392510
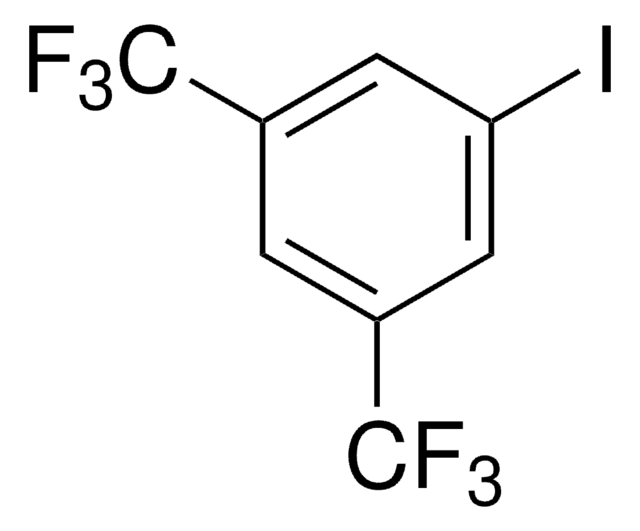
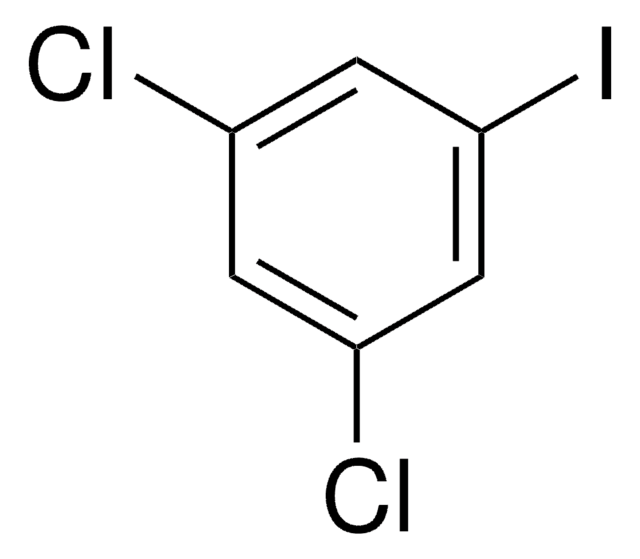
1-Iodo-3,5-dimethylbenzene
99%
Sinônimo(s):
5-Iodo-m-xylene
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
(CH3)2C6H3I
Número CAS:
Peso molecular:
232.06
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
99%
forma
liquid
índice de refração
n20/D 1.594 (lit.)
pb
92-94 °C/3 mmHg (lit.)
densidade
1.608 g/mL at 25 °C (lit.)
cadeia de caracteres SMILES
Cc1cc(C)cc(I)c1
InChI
1S/C8H9I/c1-6-3-7(2)5-8(9)4-6/h3-5H,1-2H3
chave InChI
ZLMKEENUYIUKKC-UHFFFAOYSA-N
Categorias relacionadas
Descrição geral
1-Iodo-3,5-dimethylbenzene (5-Iodo-m-xylene) is an aryl halide. It can be obtained from 5-bromo-m-xylene, via copper-catalyzed halogen exchange reaction, in the presence of NaI or KI in n-BuOH or DMF (solvents). It undergoes reaction with phenol in the presence of CuFe2O4 nano powder as a recyclable catalyst to afford 1,3-dimethyl-5-phenoxybenzene.
Aplicação
1-Iodo-3,5-dimethylbenzene (5-iodo-m-xylene) is suitable for use in the synthesis of N-(3,5-xylyl)-N-ethylaniline, an arylamine.
It may be used in the following studies:
It may be used in the following studies:
- α-Arylation of ketones.
- Copper-catalyzed N-arylation of imidazoles.
- Cyanation of 5-iodo-m-xylene to form 3,5-dimethylbenzonitrile.
- Synthesis of 1,3-Dimethyl-5-phenoxybenzene by nano-CuFe2O4 catalyzed C-O cross-coupling with phenol.
- CuBr-catalyzed amination of 1-iodo-3,5-dimethylbenzene to form N-Allyl-3,5-dimethylbenzenamine.
- Copper-catalyzed C-S bond-formation between 5-iodo-m-xylene and thiophenol.
- As a starting material in the synthesis of biphenyl-3,3′,5,5′-tetracarboxylic acid.
- Radical bromination of 5-iodo-m-xylene by N-bromosuccinimide to form 1,3-bis(bromomethyl)-5-iodobenzene.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
235.4 °F - closed cup
Ponto de fulgor (°C)
113 °C - closed cup
Equipamento de proteção individual
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Anouk S Lubbe et al.
The Journal of organic chemistry, 76(21), 8599-8610 (2011-09-21)
A study is presented on the control of rotary motion of an appending rotor unit in a light-driven molecular motor. Two new light driven molecular motors were synthesized that contain aryl groups connected to the stereogenic centers. The aryl groups
Fuk Yee Kwong et al.
Organic letters, 4(20), 3517-3520 (2002-09-27)
An efficient copper-catalyzed carbon-sulfur bond formation reaction was developed. This method is particularly noteworthy given its experimental simplicity, high generality, and exceptional level of functional group toleration and the low cost of the catalyst system. [reaction: see text]
On the synthesis of heterocyclic dendrons.
Diez-Barra E, et al.
ARKIVOC (Gainesville, FL, United States), 2002(5), 17-25 (2002)
Recyclable and reusable nano-CuFe2O4 catalyzed CO cross-coupling.
Avudoddi V, et al.
European Journal of Chemistry, 3(3), 298-304 (2012)
Recyclable and reusable nano-CuFe2O4 catalyzed CO cross-coupling.
Avudoddi V, et al.
European Journal of Organic Chemistry, 3(3), 298-304 (2012)
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica