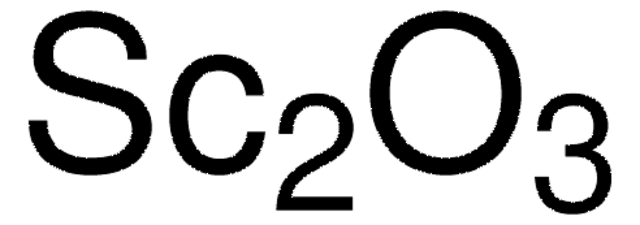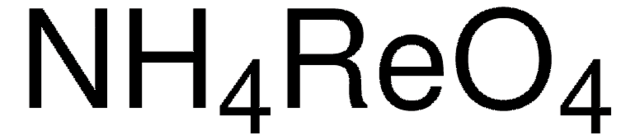294020
Scandium(III) oxide
powder, 99.995% trace rare earth metals basis
Sinônimo(s):
Scandia, Scandium trioxide
About This Item
Produtos recomendados
Nível de qualidade
Ensaio
99.995% trace rare earth metals basis
Formulário
powder
adequação da reação
core: scandium
reagent type: catalyst
cadeia de caracteres SMILES
O=[Sc]O[Sc]=O
InChI
1S/3O.2Sc
chave InChI
HYXGAEYDKFCVMU-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
Código de classe de armazenamento
13 - Non Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Artigos
Rechargeable solid-state batteries are becoming increasingly important due to wide-spread use in computers, portable electronics, and vehicular applications.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica