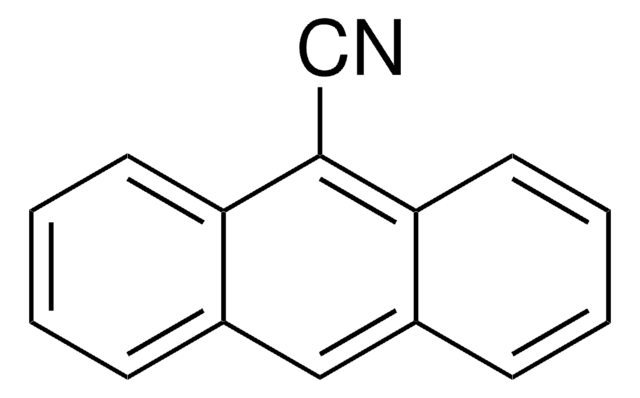
278688
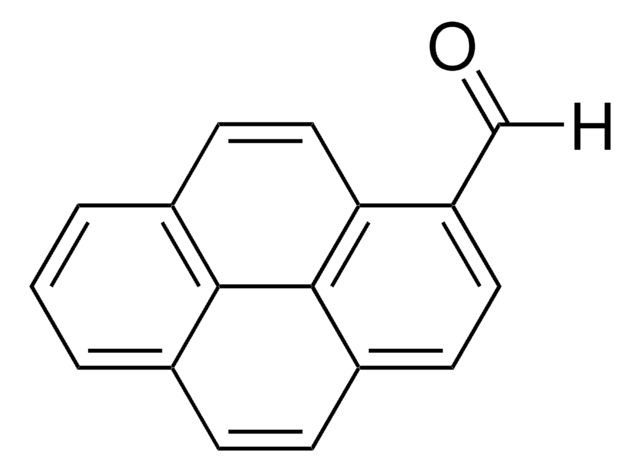
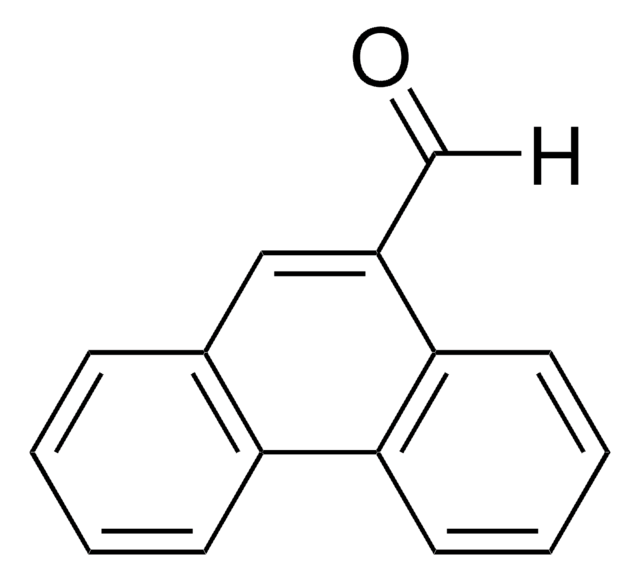
9-Anthracenecarboxaldehyde
97%
Sinônimo(s):
9-Anthraldehyde
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C15H10O
Número CAS:
Peso molecular:
206.24
Beilstein:
639167
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
97%
Formulário
solid
pf
103-105 °C (lit.)
grupo funcional
aldehyde
cadeia de caracteres SMILES
[H]C(=O)c1c2ccccc2cc3ccccc13
InChI
1S/C15H10O/c16-10-15-13-7-3-1-5-11(13)9-12-6-2-4-8-14(12)15/h1-10H
chave InChI
YMNKUHIVVMFOFO-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
The Diels-Alder reaction of 9-anthracenecarboxaldehyde with benzenediazonium-2-carboxylate was studied.
Aplicação
9-Anthracenecarboxaldehyde has been used in the synthesis of:
- new asymmetrical tridentate Schiff base ligands
- 2-(9-anthrylmethyl-ideneamino)-4-methyl-phenol, novel Schiff base via condensation with 2-amino-p-cresol
- functionalized ligand, 2-(anthracen-9-ylidene)-4,5-bis(diphenylphosphino)-4-cyclopentene-1,3-dione via Knoevenangel condensation with the diphosphine ligand 4,5-bis(diphenylphosphino)-4-cyclopentene-1,3-dione
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Knoevenagel condensation of the diphosphine ligand 4, 5-bis (diphenylphosphino)-4-cyclopentene-1, 3-dione with 9-anthracenecarboxaldehyde: Synthesis of the second-generation ligand 2-(anthracen-9-ylidene)-4, 5-bis (diphenylphosphino)-4-cyclopentene-1, 3-dione.
Watson WH, et al.
Journal of Chemical Crystallography, 36(11), 715-722 (2006)
Biswonath Biswal et al.
Dalton transactions (Cambridge, England : 2003), 46(28), 8975-8991 (2017-06-27)
A tri-fluorophoric molecular probe (1) with three different derivatized fluorophores, i.e. anthracene (An), 7-nitrobenz-2-oxa-1,3-diazole (NBD) and rhodamine-B (Rh) appended on to a Tren [tris-(2-aminoethyl)amine] receptor was demonstrated to exhibit metal ion induced ratiometric fluorescence signalling through the initiation of a
Skrollan Stockinger et al.
Beilstein journal of organic chemistry, 9, 1837-1842 (2013-09-26)
A new approach for the investigation of a higher-order reaction by on-column reaction gas chromatography is presented. The reaction and the analytical separation are combined in a single experiment to investigate the Diels-Alder reaction of benzenediazonium-2-carboxylate as a benzyne precursor
Mustafa Şahin et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 103, 400-408 (2013-01-01)
New asymmetrical tridentate Schiff base ligands were synthesized using 1,2-phenylenediamine, 4-methyl-1,2-phenylenediamine, 2-hydroxy-1-napthaldehyde, 9-anthracenecarboxaldehyde. Schiff base ligands and their metal complexes were synthesised and characterized by using FT-IR, (1)H NMR, (13)C NMR, UV-Vis, XRD, ESR, elemental analysis and fluorescence studies. The
Andrés Villalpando et al.
Acta crystallographica. Section E, Structure reports online, 66(Pt 6), o1353-o1353 (2010-01-01)
The title compound, C(22)H(17)NO, is a novel Schiff base synthesized via a condensation reaction between 9-anthracenecarboxaldehyde and 2-amino-p-cresol. The asymmetric unit contains two independent mol-ecules that are joined by an O-H⋯OH hydrogen bond. An intra-molecular O-H⋯N hydrogen bond occurs in
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 278688-100G | 4061826204757 |
| 278688-25G | 4061826204771 |
| 278688-5G | 4061826205006 |
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica