275352
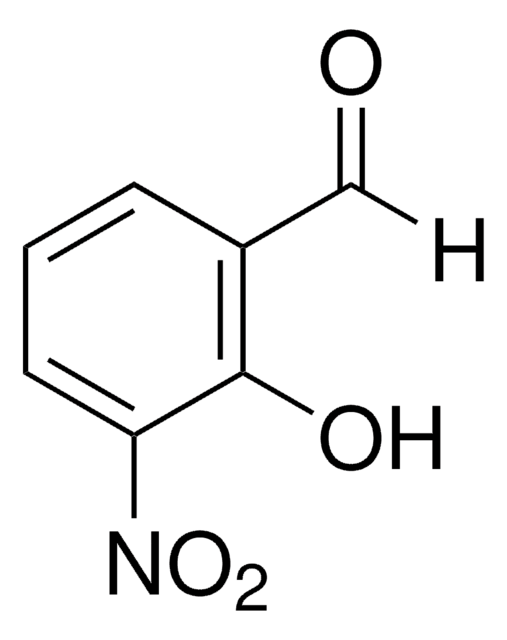
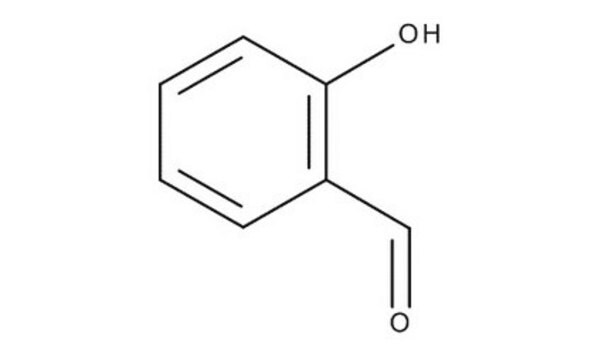
2-Hydroxy-5-nitrobenzaldehyde
98%
Sinônimo(s):
5-Nitrosalicylaldehyde
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
HOC6H3(NO2)CHO
Número CAS:
Peso molecular:
167.12
Beilstein:
512565
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
98%
pf
125-128 °C (lit.)
grupo funcional
aldehyde
nitro
cadeia de caracteres SMILES
[H]C(=O)c1cc(ccc1O)[N+]([O-])=O
InChI
1S/C7H5NO4/c9-4-5-3-6(8(11)12)1-2-7(5)10/h1-4,10H
chave InChI
IHFRMUGEILMHNU-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
2-Hydroxy-5-nitrobenzaldehyde is a nitroaromatic compound used to prepare Schiff base ligands.
The interaction of 2-hydroxy-5-nitrobenzaldehyde and chlorogenic acid (CHL) with the components of the rat hepatic glucose 6-phosphatase system was studied.
The interaction of 2-hydroxy-5-nitrobenzaldehyde and chlorogenic acid (CHL) with the components of the rat hepatic glucose 6-phosphatase system was studied.
propriedades físicas
Free of 3-nitro isomer
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Anke Rüttger et al.
BioTechniques, 41(4), 469-473 (2006-10-31)
A method is described allowing the selective determination of four cathepsins (B, H, K, and L) in live cells. Adherently growing cells are incubated with partially selective substrates for each cathepsin (peptidic derivatives of 4-methoxy-beta-naphthylamine) in microtiter plates together with
W J Arion et al.
Archives of biochemistry and biophysics, 339(2), 315-322 (1997-03-15)
We have studied the interactions of chlorogenic acid (CHL) and 2-hydroxy-5-nitrobenzaldehyde (HNB) with the components of the rat hepatic glucose 6-phosphatase (Glc-6-Pase) system. CHL and HNB are competitive inhibitors of glucose 6-phosphate (Glc-6-P) hydrolysis in intact microsomes with Ki values
M A Pajares et al.
The Journal of biological chemistry, 264(12), 6804-6809 (1989-04-25)
The 11-cis-retinal binding site of rhodopsin is of great interest because it is buried in the membrane but yet must provide an environment for charged amino acids. In addition, the active-site lysine residue must be able to engage in rapid
Anda-Mihaela Olaru et al.
Carbohydrate polymers, 179, 59-70 (2017-11-08)
A series of hydrogels based on chitosan polyamine and nitrosalicylaldehyde were prepared via dynamic covalent chemistry (DCC), by imination and transimination reactions towards ordered clusters which play the role of crosslinking nodes of the chitosan network. The hydrogelation mechanism has
Anas G Elsafy et al.
Sensors (Basel, Switzerland), 18(7) (2018-07-13)
(E)-2-((benzo[d]thiazol-2-ylimino)methyl)-4-nitrophenol 1 and (E)-2-(((6-methoxybenzo[d]thiazol-2-yl)imino)methyl)-4-nitrophenol 2 were synthesized efficiently under microwave conditions. The structures were confirmed using IR, ¹H NMR, and 13C NMR. UV-vis. Fluorescence investigations demonstrated that 1 and 2 are sensitive and selective sensors for detection of cyanide over
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 275352-1G | |
| 275352-25G | 4061826166673 |
| 275352-5G | 4061826166680 |
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica