252832
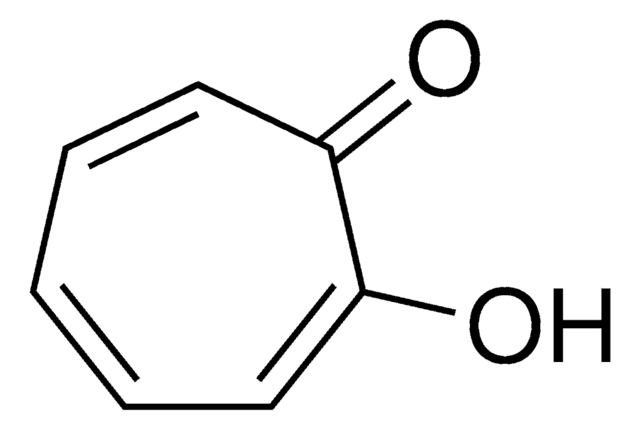
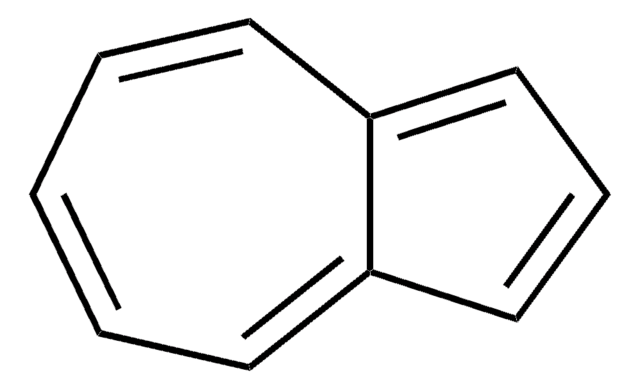
Tropone
97%
Sinônimo(s):
2,4,6-Cycloheptatrien-1-one
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C7H6O
Número CAS:
Peso molecular:
106.12
Beilstein:
1902335
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
97%
Formulário
liquid
índice de refração
n20/D 1.615 (lit.)
p.e.
113 °C/15 mmHg (lit.)
densidade
1.094 g/mL at 25 °C (lit.)
grupo funcional
ketone
temperatura de armazenamento
−20°C
cadeia de caracteres SMILES
O=C1C=CC=CC=C1
InChI
1S/C7H6O/c8-7-5-3-1-2-4-6-7/h1-6H
chave InChI
QVWDCTQRORVHHT-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Metal-catalyzed [6+3] cycloaddition of tropone with azomethine ylides has been reported.
Aplicação
Tropone has been used in synthesis of:
- bicyclic δ-lactones via heterocyclic carbine-catalyzed [8+3] annulation pathway
- 6,7-benzobicyclo [3.2.2] nona-3,6,8-trien-2-one via thermal addition to bezyne
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Marie Varin et al.
The Journal of organic chemistry, 72(17), 6421-6426 (2007-07-28)
In this paper we report the rearrangement of spirocyclohexadienones into dihydrotropones in basic conditions as a new method for the preparation of seven-membered ring ketones, which are key building blocks for the synthesis of tropoloalkaloids. DFT calculations and deuterium labeling
Barry M Trost et al.
Organic letters, 11(16), 3782-3785 (2009-07-18)
A concise approach to the core skeleton of the welwitindolinone alkaloids was developed on the basis of sequential cycloaddition reactions. First, a palladium catalyzed enantioselective [6 + 3] trimethylenemethane cycloaddition onto a tropone nucleus was used to generate the requisite
Honglei Liu et al.
Journal of the American Chemical Society, 136(6), 2625-2629 (2014-01-24)
The first metal-catalyzed [6 + 3] cycloaddition of tropone with azomethine ylides has been developed. With the use of a chiral ferrocenylphosphine-copper(I) complex as the catalyst, the asymmetric variant of the [6 + 3] cycloaddition has also been successfully achieved.
Strahil Berkov et al.
Zeitschrift fur Naturforschung. C, Journal of biosciences, 59(3-4), 184-186 (2004-07-10)
The alkaloid spectra of Datura innoxia plants grown in Egypt and Bulgaria were investigated by GC-MS. Thirty-eight alkaloids were detected in the roots, leaves and fruits of the plants. Five new alkaloids for D. innoxia are reported. Alkaloid spectra of
Young-Sun Do et al.
The Journal of organic chemistry, 74(2), 917-920 (2008-12-05)
A ring-expansion protocol that consisted of the 1,2-addition of various enolate nucleophiles to 6-trimethylsiloxy-2-cyclohexene-1-one (1) and the NaIO(4)-promoted oxidative ring opening of the resulting diols 2, followed by an intramolecular Knoevenagel condensation, furnished versatile dihydrotropones 6. Maintaining Z-configuration in the
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica