251062
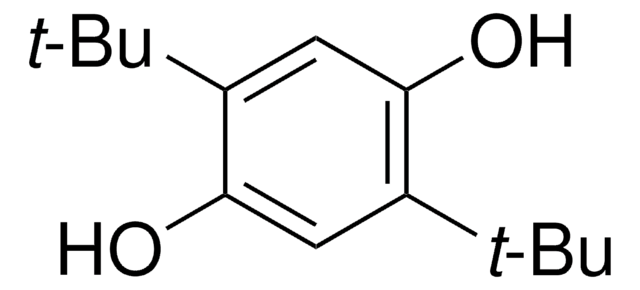
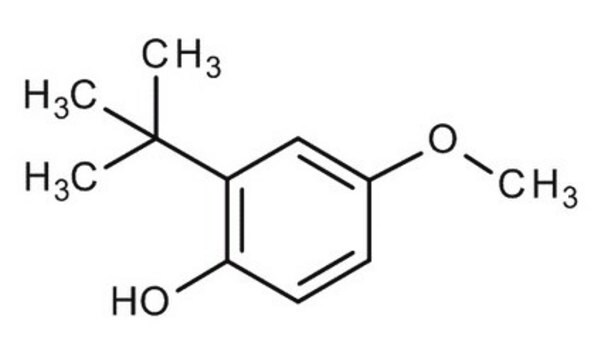
2,6-Di-tert-butyl-4-methoxyphenol
97%
Sinônimo(s):
3,5-Di-tert-butyl-4-hydroxyanisole
About This Item
Produtos recomendados
Ensaio
97%
pf
102-106 °C (lit.)
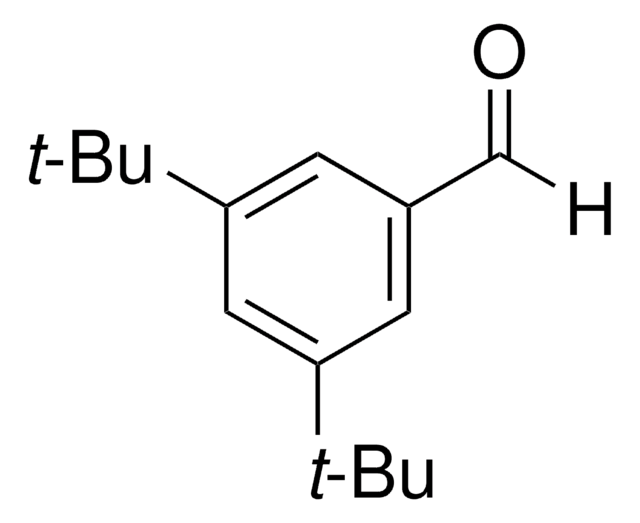
cadeia de caracteres SMILES
COc1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C
InChI
1S/C15H24O2/c1-14(2,3)11-8-10(17-7)9-12(13(11)16)15(4,5)6/h8-9,16H,1-7H3
chave InChI
SLUKQUGVTITNSY-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica