230367
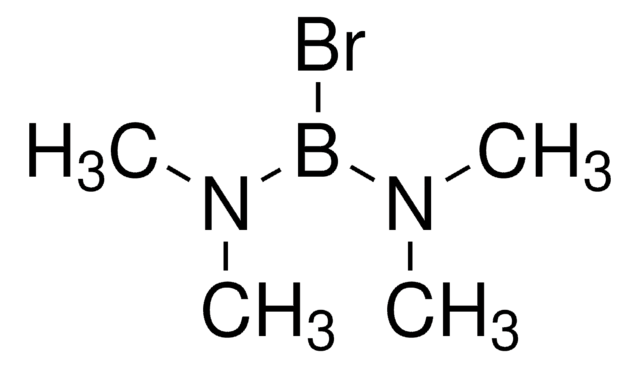
Boron tribromide
≥99.99%
Sinônimo(s):
Tribromoboron
About This Item
Produtos recomendados
densidade de vapor
8.6 (vs air)
Nível de qualidade
pressão de vapor
40 mmHg ( 14 °C)
Ensaio
≥99.99%
Formulário
liquid
p.e.
~90 °C (lit.)
pf
−46 °C (lit.)
densidade
2.60 g/mL at 20 °C (lit.)
cadeia de caracteres SMILES
BrB(Br)Br
InChI
1S/BBr3/c2-1(3)4
chave InChI
ILAHWRKJUDSMFH-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Aplicação
- Drug intermediate 6-nitro-L-DOPA
- Luminescent polystyrene derivatives with sterically protected carbazolylborane moieties
- High-quality boron-doped graphene via Wurtz-type reductive coupling reaction
- Mercapto-(+)-methamphetamine haptens for synthesis of (+)-methamphetamine conjugate vaccines with improved epitope densities
- Micrometer-sized organic molecule-DNA hybrid structures
- Borane complexes via electrophilic aromatic borylation reactions
- A 5-HT2C receptor agonist
- Biphenyl-derivatives possessing tertiary amino groups as β-secretase(BACE1) inhibitors for the treatment of Alzheimer′s disease
- A highly near-IR region fluorescent p-extended boron aza-dipyrromethene moiety unit
- Tetrahydroisoquinoline derivatives via intramolecular cyclization of methoxy-substituted N-phenethylimides
acessório
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 2 Inhalation - Acute Tox. 2 Oral - Eye Dam. 1 - Skin Corr. 1A
Código de classe de armazenamento
6.1B - Non-combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe de risco de água (WGK)
WGK 3
Equipamento de proteção individual
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 230367-100G | 4061838125798 |
| 230367-25G | 4061838782595 |
| 230367-5G | 4061838782601 |
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica