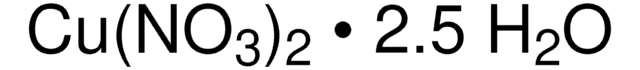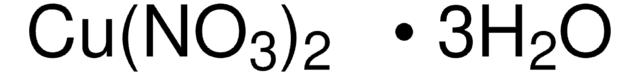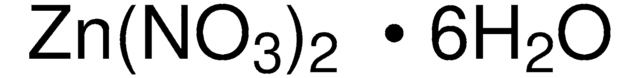203874
Nickel(II) nitrate hexahydrate
99.999% trace metals basis
Sinônimo(s):
Nickel dinitrate hexahydrate, Nickel nitrate hexahydrate, Nickel(2+) dinitrate hexahydrate, Nickelous nitrate hexahydrate
About This Item
Produtos recomendados
grau
for analytical purposes
Nível de qualidade
Ensaio
99.999% trace metals basis
Formulário
solid
Impurezas
≤15.0 ppm Trace Metal Analysis
pf
56 °C (lit.)
densidade
2.05 g/mL at 25 °C (lit.)
aplicação(ões)
battery manufacturing
cadeia de caracteres SMILES
O.O.O.O.O.O.[Ni++].[O-][N+]([O-])=O.[O-][N+]([O-])=O
InChI
1S/2NO3.Ni.6H2O/c2*2-1(3)4;;;;;;;/h;;;6*1H2/q2*-1;+2;;;;;;
chave InChI
AOPCKOPZYFFEDA-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Aplicação
In addition, it can be used:
- As an additive in the fabrication of cellulose acetate polymers(CA) to control the porosity of the polymer. The formation of Ni(NO3)2.6H2O aggregates in the polymer matrix during solidification and the strong interaction between Ni, nitrate, and water molecules results in the formation of well-defined pores on the surface of the CA matrix.
- As a starting material to prepare nickel(ii) Schiff base, which is used as a precursor to synthesize NiO nanoparticles by solid-state thermal decomposition method.
- As a dopant to prepare Ni-Ceria catalyst for selective hydrogenation of acetylene.
- As a catalyst to prepare 3,4-dihydropyrimidinone derivatives via Biginelli cyclocondensation.
Características e benefícios
- Exceptional Purity: The 99.999% purity of Nickel(II) nitrate hexahydrate minimizes contamination from trace metals, ensuring suitability for applications sensitive to even minute impurities.
- Consistent Performance: Ultra-high purity guarantees consistent performance across various applications, reducing variability and enhancing reliability.
- High Purity Standard: Ideal as a standard or reagent for trace metal analysis and high-precision analytical techniques, ensuring accurate and reliable results.
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1A - Eye Dam. 1 - Muta. 2 - Ox. Sol. 2 - Repr. 1B - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT RE 1 Inhalation
Código de classe de armazenamento
5.1B - Oxidizing hazardous materials
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
Lithium-Ion Battery Performance: Dependence on Material Synthesis and Post‑Treatment Methods
The prevailing strategies for heat and electric-power production that rely on fossil and fission fuels are having a negative impact on the environment and on our living conditions.
Plasmonic nanoparticles have unique optical properties that can be tailored to suit a variety of applications in the biotechnology1–8 and electronics9–16 industries.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| PH010596-1EA | |
| 203874-20G | 4061838766434 |
| 203874-100G | 4061838766427 |
| 203874-500G | 4061838766441 |
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica