193291
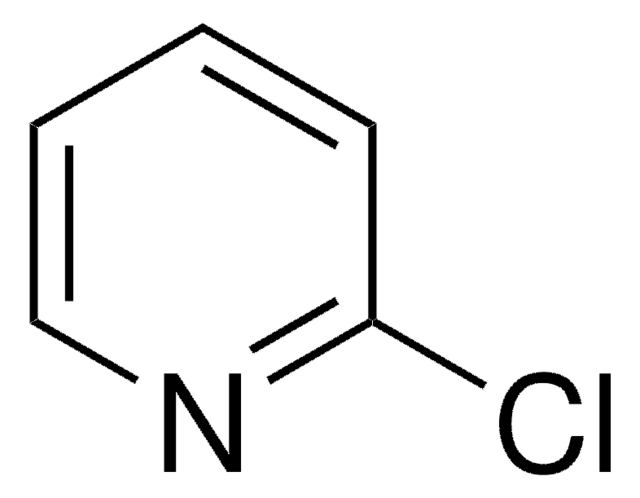
2-Chloropyrimidine
95%
Sinônimo(s):
2-Chloro-1,3-pyrimidine, Pyrimidin-2-yl chloride
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C4H3ClN2
Número CAS:
Peso molecular:
114.53
Beilstein:
107171
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
95%
Formulário
crystals
p.e.
75-76 °C/10 mmHg (lit.)
pf
63-66 °C (lit.)
grupo funcional
chloro
cadeia de caracteres SMILES
Clc1ncccn1
InChI
1S/C4H3ClN2/c5-4-6-2-1-3-7-4/h1-3H
chave InChI
UNCQVRBWJWWJBF-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
2-Chloropyrimidine undergoes cobalt-catalyzed cross-coupling reaction with aryl halides.
Aplicação
2-Chloropyrimidine was used in the synthesis of:
- novel bis(2-(pyrimidin-2-yl)ethoxy)alkanes
- 4′-(1,1′-(5-(2-methoxyphenoxy)-[2,2′-bipyrimidine]-4,6-diyl)bis(1H-pyrazol-3,1-diyl)) dianiline fluorescent dye, biosensor for protein assay
- cis- and trans-octahydropyrrolo[2,3]pyridine derivatives
- 2-amino-4-heteroarylpyrimidines
Outras notas
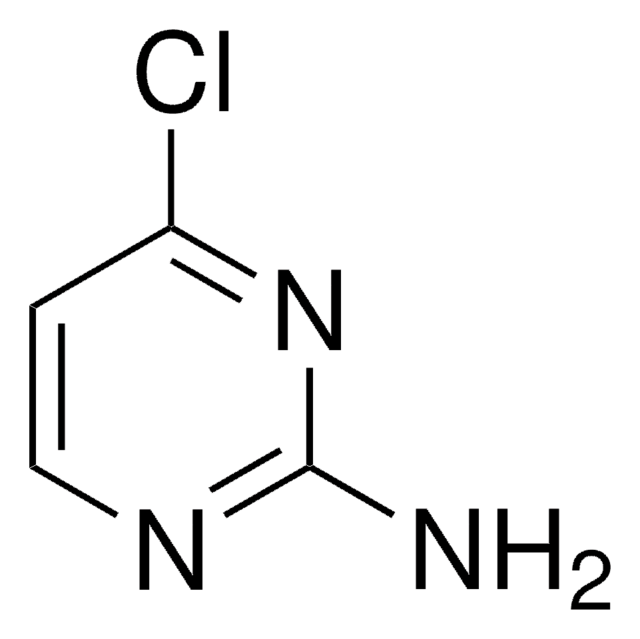
Remainder 2-hydroxypyrimidine
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral - Eye Irrit. 2
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
208.4 °F - closed cup
Ponto de fulgor (°C)
98 °C - closed cup
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Vangavaragu Jhansi Rani et al.
Archiv der Pharmazie, 345(8), 663-669 (2012-05-18)
The pyrimidine nucleus is an important component of nucleic acids (DNA and RNA) and vitamins (B(2) and folic acid). It is evident from the literature that pyrimidine derivatives possess a wide spectrum of biological activities such as antioxidant, anticancer, antibacterial
Matthew G Bursavich et al.
Organic letters, 7(19), 4113-4116 (2005-09-09)
[reaction: see text] An expedient synthesis of diverse 2-amino-4-heteroarylpyrimidines via a 2-chloropyrimidine intermediate is described. A series of potentially biologically active analogues have been synthesized in two parallel steps to afford focused arrays.
Igor Goljer et al.
Chirality, 21(7), 681-691 (2008-09-17)
Reaction of (S)- or (R)-3-aminoquinuclidine with 2-chloropyrimidine or 2-bromopyrimidine led to an unexpected formation of both cis- and trans-octahydropyrrolo [2,3]pyridine derivatives. A single-step synthesis of two of the four stereoisomers of these octahydropyrrolo[2,3]pyridine derivatives provides a convenient way of generating
Vikas S Padalkar et al.
Chemistry Central journal, 5, 72-72 (2011-11-10)
Fluorescent dyes with biocompatible functional group and good fluorescence behavior are used as biosensor for monitoring different biological processes as well as detection of protein assay. All reported fluorophore used as sensors are having high selectivity and sensitivity but till
Carina Sollert et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 21(14), 5380-5386 (2015-02-18)
The Ru-catalysed C2-H arylation of indoles and pyrroles by using boronic acids under oxidative conditions is reported. This reaction can be applied to tryptophan derivatives and tolerates a wide range of functional groups on both coupling partners, including bromides and
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica