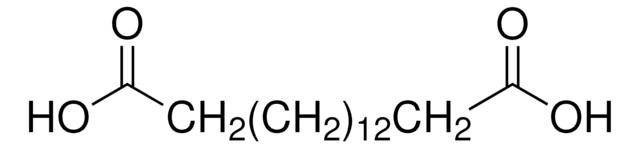177490
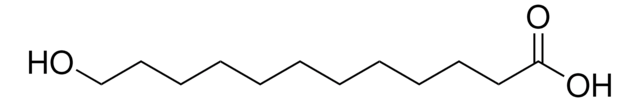
16-Hydroxyhexadecanoic acid
98%
Sinônimo(s):
16-Hydroxypalmitic acid, Juniperic acid
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
HO(CH2)15CO2H
Número CAS:
Peso molecular:
272.42
Beilstein:
1783998
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
98%
Formulário
solid
pf
94-98 °C (lit.)
grupo funcional
carboxylic acid
hydroxyl
cadeia de caracteres SMILES
OCCCCCCCCCCCCCCCC(O)=O
InChI
1S/C16H32O3/c17-15-13-11-9-7-5-3-1-2-4-6-8-10-12-14-16(18)19/h17H,1-15H2,(H,18,19)
chave InChI
UGAGPNKCDRTDHP-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Aplicação
16-Hydroxyhexadecanoic acid was used in the synthesis of dihydroxypalmitic acids. It was also used to induce the expression of two GRP genes of Arabidopsis thaliana, AtGRP5 and AtGRP23.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Sapa Hima Rani et al.
The Journal of biological chemistry, 285(49), 38337-38347 (2010-10-06)
A key step in the triacylglycerol (TAG) biosynthetic pathway is the final acylation of diacylglycerol (DAG) by DAG acyltransferase. In silico analysis has revealed that the DCR (defective in cuticular ridges) (At5g23940) gene has a typical HX(4)D acyltransferase motif at
Akihisa Abe et al.
Journal of biochemistry, molecular biology, and biophysics : JBMBB : the official journal of the Federation of Asian and Oceanian Biochemists and Molecular Biologists (FAOBMB), 6(1), 37-43 (2002-08-21)
We have found that omega-hydroxy palmitic acid (16-hydroxy palmitic acid, omega-HPA) has both cell growth inhibiting and cell death inducing actions on human lung adenosquamous carcinoma cell line H596 and adenocarcinoma cell line A549. Further, these effects were dose- and
Jong Ho Park et al.
Plant physiology and biochemistry : PPB, 46(11), 1015-1018 (2008-07-29)
Glycine-rich proteins (GRPs) belong to a large family of heterogenous proteins that are enriched in glycine residues. The expression of two GRP genes of Arabidopsis thaliana, AtGRP5 and AtGRP23, was induced by 16-hydroxypalmitic acid (HPA), a major component of cutin.
G D Rees et al.
Biochimica et biophysica acta, 1257(3), 239-248 (1995-08-03)
Five microbial lipases from Chromobacterium viscosum, Candida cylindracea, Pseudomonas (source Fluka), Pseudomonas (source Genzyme) and lipoprotein lipase ex Microbial (Genzyme) have been screened for lactonisation activity towards 16-hydroxyhexadecanoic acid (HHA) in a variety of different w/o microemulsion systems. With the
Jean-Paul Douliez
Journal of colloid and interface science, 271(2), 507-510 (2004-02-20)
The interaction between cutin and suberin monomers, i.e., omega -hydroxylpalmitic acid, alpha, omega -hexadecanedioic acid, alpha, omega --hexadecanediol, 12-hydroxylstearic acid, and phospholipid vesicles biomimicking the lipid structure of plant cell membranes has been studied by optical and transmission electron microscopy
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica