157163
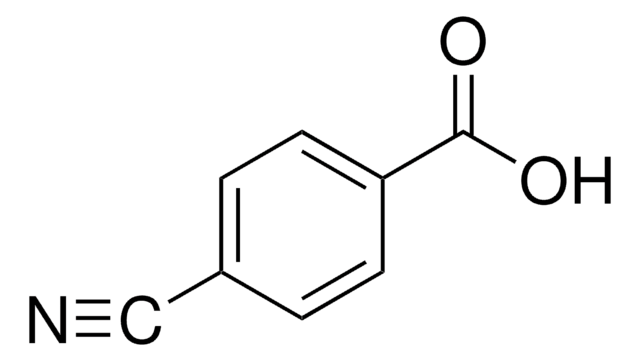
3-Cyanobenzoic acid
98%
Sinônimo(s):
Isophthalic acid mononitrile
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
NCC6H4CO2H
Número CAS:
Peso molecular:
147.13
Beilstein:
1862566
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
98%
forma
powder
pf
220-224 °C (lit.)
grupo funcional
carboxylic acid
cadeia de caracteres SMILES
OC(=O)c1cccc(c1)C#N
InChI
1S/C8H5NO2/c9-5-6-2-1-3-7(4-6)8(10)11/h1-4H,(H,10,11)
chave InChI
GYLKKXHEIIFTJH-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
3-Cyanobenzoic acid was used in the preparation of new Co(II)-doped Zn(II)-tetrazole-benzoate coordination polymers via in situ [2+3] cycloaddition reactions with NaN3 in the presence of Zn(II) and/or Co(II) salts under hydrothermal conditions. It was also used in the synthesis of three-dimensional coordination polymer, [Mn3(OH)2Na2(3-cnba)6]n (3-Hcnba = 3-cyanobenzoic acid).
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Wei-Chao Song et al.
Inorganic chemistry, 48(8), 3792-3799 (2009-03-24)
In our continuing efforts to explore the effects of ligand modifications on the structures and properties of their metal complexes, we studied the in situ [2 + 3] cycloaddition reactions of benzonitrile, o-phthalodinitrile, 3-cyanobenzoic acid, 4-cyanobenzoic acid with NaN(3) in
Jin-Tang Li et al.
Inorganic chemistry, 44(13), 4448-4450 (2005-06-21)
A three-dimensional coordination polymer, [Mn3(OH)2Na2(3-cnba)6]n (1) (3-Hcnba = 3-cyanobenzoic acid), has been synthesized by the reaction of MnCl2, NaN3, and 3-Hcnba in water. Its crystal structure was determined by single-crystal X-ray diffraction. Magnetic studies show that the complex behaves as
Tomoko Abe et al.
The Journal of antibiotics, 70(4), 435-442 (2016-10-13)
The adenylation domain of nonribosomal peptide synthetase (NRPS) is responsible for the selective substrate recognition and its activation (as an acyl-O-AMP intermediate) during ATP consumption. DhbE, a stand-alone adenylation domain, acts on an aromatic acid, 2,3-dihydroxybenzoic acid (DHB). This activation
Homan Kang et al.
Scientific reports, 5, 10144-10144 (2015-05-29)
Recently, preparation and screening of compound libraries remain one of the most challenging tasks in drug discovery, biomarker detection, and biomolecular profiling processes. So far, several distinct encoding/decoding methods such as chemical encoding, graphical encoding, and optical encoding have been
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica