139432
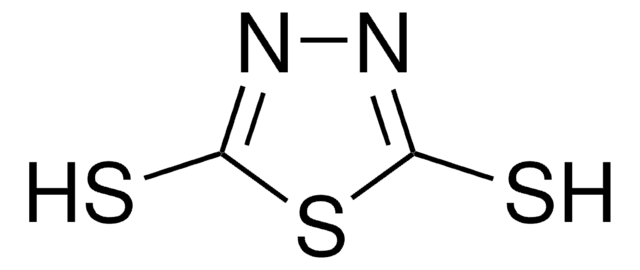
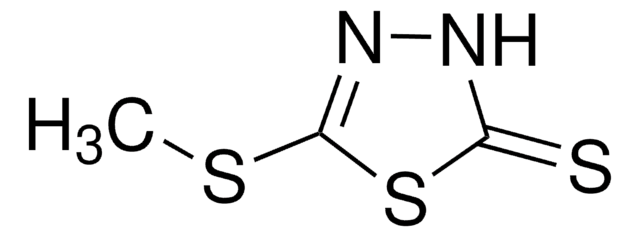
1,3,4-Thiadiazole-2,5-dithiol dipotassium salt
98%
Sinônimo(s):
Bismuthiol I dipotassium salt, 2,5-Dimercapto-1,3,4-thiadiazole dipotassium salt, Bismuththiol I dipotassium salt
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C2K2N2S3
Número CAS:
Peso molecular:
226.43
Beilstein:
4917786
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
98%
pf
274-276 °C (dec.) (lit.)
cadeia de caracteres SMILES
[K]Sc1nnc(S[K])s1
InChI
1S/C2H2N2S3.2K/c5-1-3-4-2(6)7-1;;/h(H,3,5)(H,4,6);;/q;2*+1/p-2
chave InChI
GPWLFGDMYSVEGN-UHFFFAOYSA-L
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
1,3,4-thiadiazole-2,5-dithiol undergoes copolymerization with 1+, 2+ and 3+ valent metals to form coordination polymers. It forms polymers with metals and can be useful in treatment of metal-polluted water.
Aplicação
1,3,4-thiadiazole-2,5-dithiol (Bismuthiol I) was used in determination of tellurium in indium phosphide doped with cadmium telluride by electrothermal atomic absorption spectrometry.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Determination of tellurium in indium phosphide by electrothermal atomic absorption spectrometry and ultraviolet-visible spectrophotometry.
Taddia M, et al.
Journal of Analytical Atomic Spectrometry, 10(6), 433-437 (1995)
Synthesis of metallic azoderivatives of 2-amino-5-mercapto-1, 3, 4-thiadiazole.
Ortega-Luoni P, et al.
Journal of the Chilean Chemical Society, 52(1), 1120-1122 (2007)
Coordination polymers from 1, 3, 4-thiadiazole-2, 5-dithiol and metal ions.
Ortega P, etal.
Macromolecular Chemistry and Physics, 198(9), 2949-2956 (1997)
N N Ugarova et al.
Biokhimiia (Moscow, Russia), 45(12), 2243-2253 (1980-12-01)
The individual and combined oxidation of 5-mercapto-1,3,4-thiadiazolthione-2 (bismuthol I) and 3,3'-dimethoxybenzidine (o-dianisidine) by hydrogen peroxide catalyzed by horseradish peroxidase (pH 5,0) was studied. It was shown that bismuthol I is a substrate for peroxidase, which is competitive towards o-dianisidine. In
M Jamaluddin Ahmed et al.
Analytical sciences : the international journal of the Japan Society for Analytical Chemistry, 18(7), 805-810 (2002-07-26)
A simple spectrophotometric method is presented for the rapid determination of copper at a trace level using 2,5-dimercapto-1,3,4-thiadiazole (DMTD) as a new spectrophotometric reagent. The method is based on the reaction of non-absorbent DMTD in a slightly acidic (0.002-0.014 mol
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica