132020
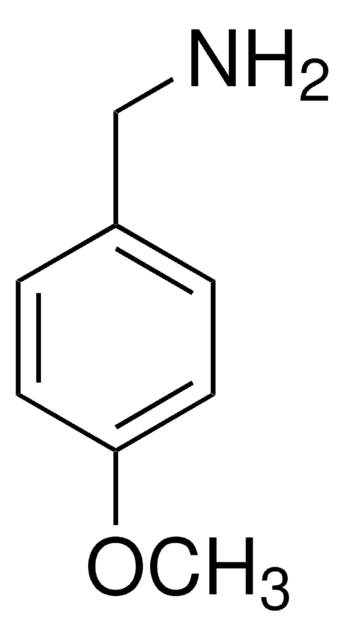
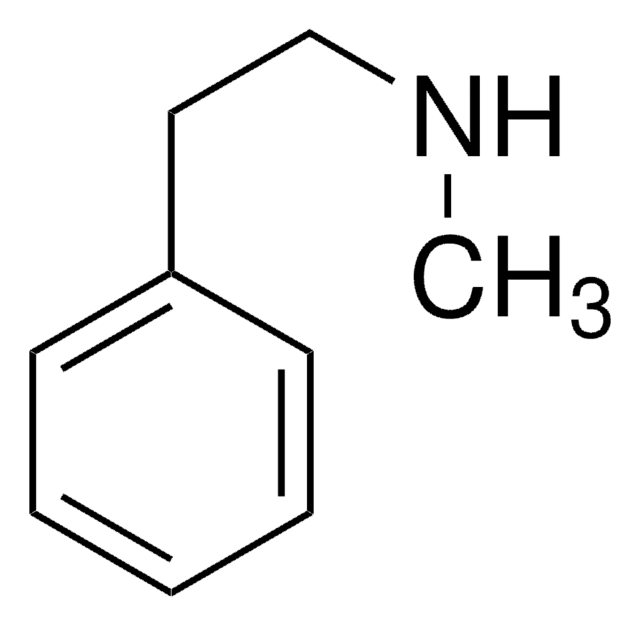
2-(p-Tolyl)ethylamine
97%
Sinônimo(s):
4-Methylphenethylamine
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
CH3C6H4CH2CH2NH2
Número CAS:
Peso molecular:
135.21
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
97%
Formulário
liquid
índice de refração
n20/D 1.527 (lit.)
p.e.
214 °C (lit.)
densidade
0.93 g/mL at 25 °C (lit.)
grupo funcional
amine
cadeia de caracteres SMILES
Cc1ccc(CCN)cc1
InChI
1S/C9H13N/c1-8-2-4-9(5-3-8)6-7-10/h2-5H,6-7,10H2,1H3
chave InChI
VKJXAQYPOTYDLO-UHFFFAOYSA-N
Informações sobre genes
human ... CYP1A2(1544)
Categorias relacionadas
Aplicação
2-(p-Tolyl)ethylamine was used to prepare secondary amides by amidation of sophorolipid ethyl ester.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
195.8 °F - closed cup
Ponto de fulgor (°C)
91 °C - closed cup
Equipamento de proteção individual
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Sanjay K Singh et al.
The Journal of organic chemistry, 68(14), 5466-5477 (2003-07-04)
Novel enzyme-mediated synthetic routes were developed to provide a new family of sophorolipid derivatives and glycolopid-based amphiphilic monomers. These compounds are of great interest for their potential use in immunoregulation, as well as for other biological properties. In the present
Anita H Lewin et al.
Bioorganic & medicinal chemistry, 16(15), 7415-7423 (2008-07-08)
A cell line in which RD-HGA16 cells were stably transfected with the hTAAR 1 receptor was created and utilized to carry out a systematic evaluation of a series of beta-phenethylamines. Fair agreement was observed with data obtained for aryl and
Laura E Korhonen et al.
Journal of medicinal chemistry, 48(11), 3808-3815 (2005-05-27)
The purpose of this study was to determine the cytochrome P450 1A2 (CYP1A2) inhibition potencies of structurally diverse compounds to create a comprehensive three-dimensional quantitative structure-activity relationship (3D-QSAR) model of CYP1A2 inhibitors and to use this model to predict the
Minna Rahnasto et al.
Journal of medicinal chemistry, 48(2), 440-449 (2005-01-22)
The purpose of this study was to develop screening and in silico modeling methods to obtain accurate information on the active center of CYP2A6, a nicotine oxidizing enzyme. The inhibitory potencies of 26 naphthalene and 16 non-naphthalene derivatives were determined
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica