12268
Ethyl benzimidate hydrochloride
≥97.0% (AT)
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
C6H5C(=NH)OCH2CH3 · HCl
Número CAS:
Peso molecular:
185.65
Beilstein:
3913195
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
≥97.0% (AT)
pf
~125 °C (dec.)
grupo funcional
ether
phenyl
cadeia de caracteres SMILES
Cl[H].CCOC(=N)c1ccccc1
InChI
1S/C9H11NO.ClH/c1-2-11-9(10)8-6-4-3-5-7-8;/h3-7,10H,2H2,1H3;1H
chave InChI
MODZVIMSNXSQIH-UHFFFAOYSA-N
Categorias relacionadas
Descrição geral
Ethyl benzimidate hydrochloride reacts with (R)-ethyl cysteine hydrochloride in ethanol to yield (4R)-ethyl 2-phenyl-4,5-dihydrothiazole-4-carboxylate. It reacts with D-Penicillamine methyl ester hydrochloride and triethylamine to yield methyl-5,5- dimethyl-2-phenyl-2-thiazoline-4-carboxylate.
Aplicação
Intermediate for synthesis
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
The Synthesis of Substituted Penicillins and Simpler Structural Analogs. III. Phthalimido ?-Lactam-Thiazolidines Derived from Penicillamine.
Sheehan JC, et al.
Journal of the American Chemical Society, 73(9), 4373-4375 (1951)
Satendra Singh et al.
The Journal of organic chemistry, 69(13), 4551-4554 (2004-06-19)
(1R)-(+)-2,10- and (1S)-(-)-2,10-camphorsultam were acylated with ethyl 2-phenylthiazoline 4-carboxylate to afford (+)- and (-)-2-phenylthiazolinylcamphorsultam, which were stereoselectively alkylated with MeI in the presence of n-BuLi. Alkylation of these phenylthiazolinylcamphorsultams occurred from the beta-face rather than alpha-face, resulting in the formation
Aerobic dissipation of the novel cyanoacrylate fungicide phenamacril in soil and sludge incubations.
Søren S Donau et al.
Chemosphere, 233, 873-878 (2019-07-26)
The cyanoacrylate ethyl (2Z)-3-amino-2-cyano-3-phenylacrylate (phenamacril), has been introduced as an effective agent against several fungi species belonging to the Fusarium genus. However, in current literature, knowledge about the environmental behavior of this fungicide is limited and there are no data
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica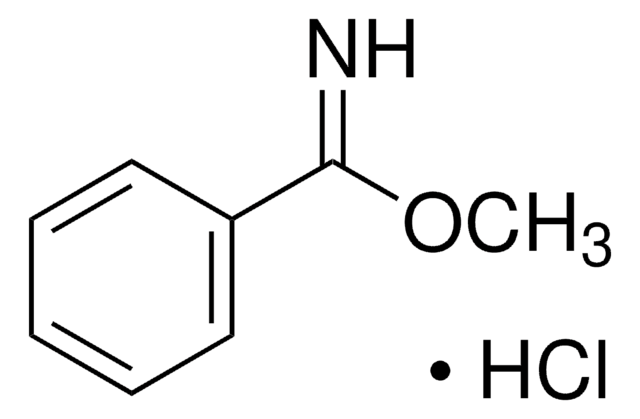

![4,7,13,16,21,24-Hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane 98%](/deepweb/assets/sigmaaldrich/product/structures/189/812/8a6555e5-8de6-4236-865f-19339cee3634/640/8a6555e5-8de6-4236-865f-19339cee3634.png)