117757
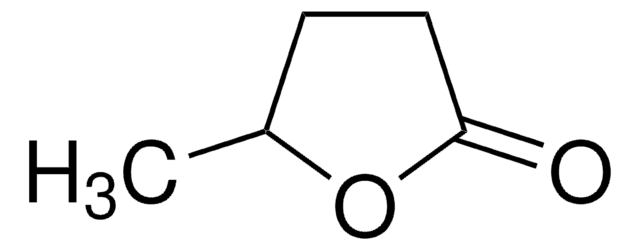
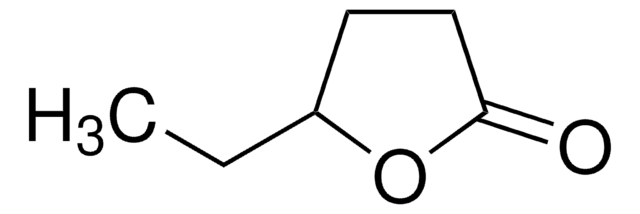
α-Methyl-γ-butyrolactone
98%
Sinônimo(s):
4,5-Dihydro-3-methyl-2(3H)-furanone
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C5H8O2
Número CAS:
Peso molecular:
100.12
Beilstein:
80418
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
98%
índice de refração
n20/D 1.432 (lit.)
p.e.
78-81 °C/10 mmHg (lit.)
solubilidade
THF: soluble
grupo funcional
ester
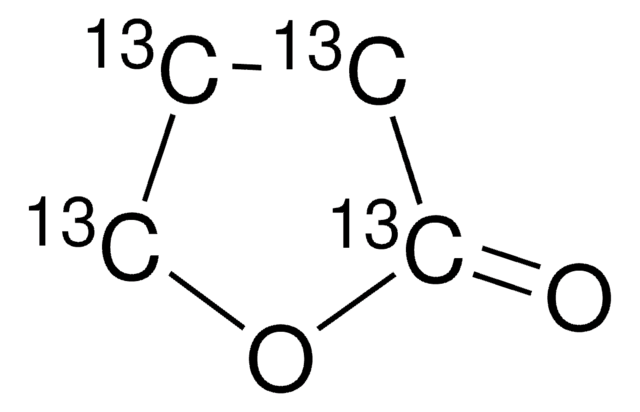
cadeia de caracteres SMILES
CC1CCOC1=O
InChI
1S/C5H8O2/c1-4-2-3-7-5(4)6/h4H,2-3H2,1H3
chave InChI
QGLBZNZGBLRJGS-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
α-Methyl-γ-butyrolactone undergoes benzylation to give racemic α-benzyl-α-methyl-γ-butyrolactone.
Aplicação
α-Methyl-γ-butyrolactone was used as model compound in Bracketing experiments to investigate the thermodynamically favored site of reaction of pilocarpine.
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
163.4 °F - closed cup
Ponto de fulgor (°C)
73 °C - closed cup
Equipamento de proteção individual
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
M Satterfield et al.
Journal of the American Society for Mass Spectrometry, 10(3), 209-216 (1999-03-09)
Analysis of the sites of reaction of a biologically important compound, pilocarpine, a molecule with imidazole and butyrolactone rings connected by a methylene bridge, has been accomplished in a quadrupole ion trap with the aim of characterizing its structure/reactivity relationships.
Eric B Gonzales et al.
The Journal of pharmacology and experimental therapeutics, 309(2), 677-683 (2004-01-27)
Alkyl-substituted butyrolactones have both inhibitory and stimulatory effects on GABA(A) receptors. Lactones with small alkyl substitutions at the alpha-position positively modulate the channel, whereas beta-substituted lactones tend to inhibit the GABA(A) receptor. These compounds mediate inhibition through the picrotoxin site
Hagai Tavori et al.
Bioorganic & medicinal chemistry, 16(15), 7504-7509 (2008-06-24)
Paraoxonase1 (PON1) is a HDL bound enzyme and many of the anti-atherogenic properties of HDL are attributed to PON1. The enzyme precise mechanism of protective action and its endogenous substrate remain elusive. PON1 hydrolyzes organophosphates, arylesters and lactones, whereas the
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica