22620
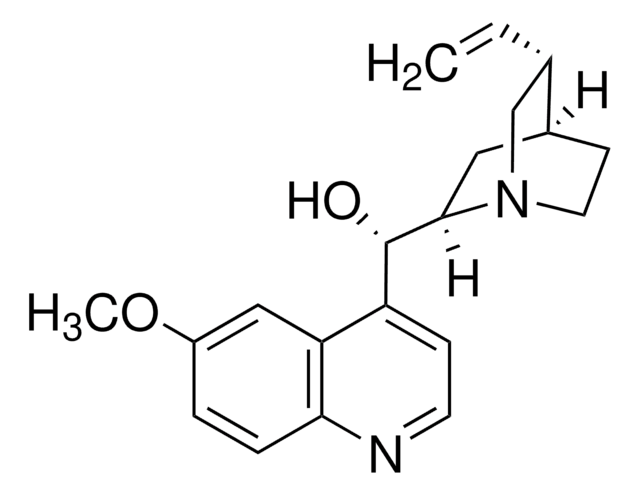
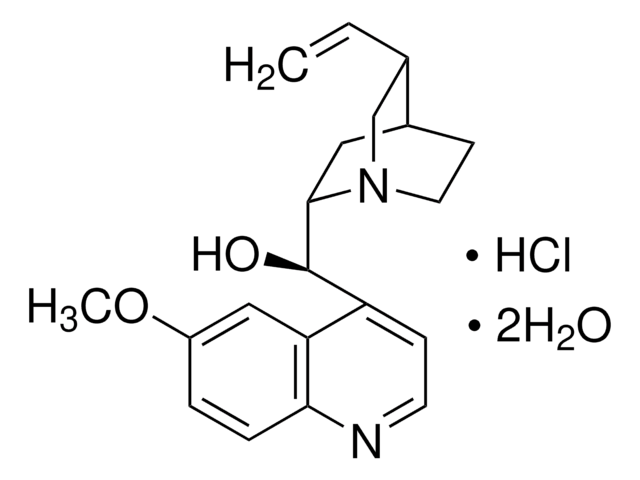
Quinine
suitable for fluorescence, anhydrous, ≥98.0% (dried material, NT)
Synonyme(s) :
6′-Methoxycinchonidine
About This Item
Produits recommandés
Niveau de qualité
Pureté
≥98.0% (dried material, NT)
Forme
powder
Activité optique
[α]20/D −126±5°, c = 1% in chloroform
Impuretés
≤5% dihydroquinine (HPLC)
Perte
≤1% loss on drying, 110 °C
Pf
173-175 °C (lit.)
Solubilité
H2O: soluble
Fluorescence
λex 347 nm; λem 448 nm in 0.5 M sulfuric acid
Adéquation
suitable for fluorescence
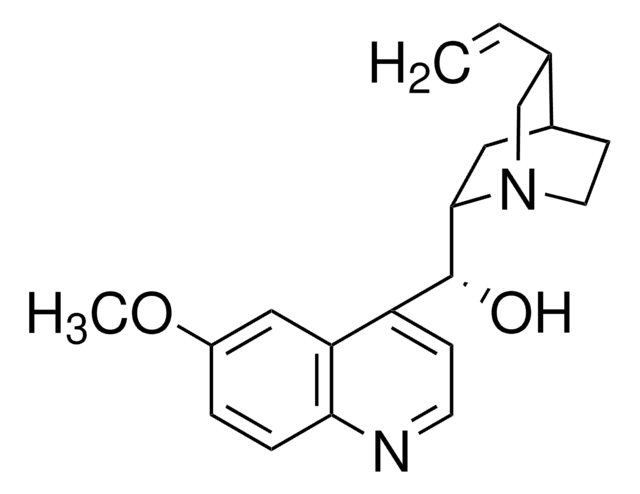
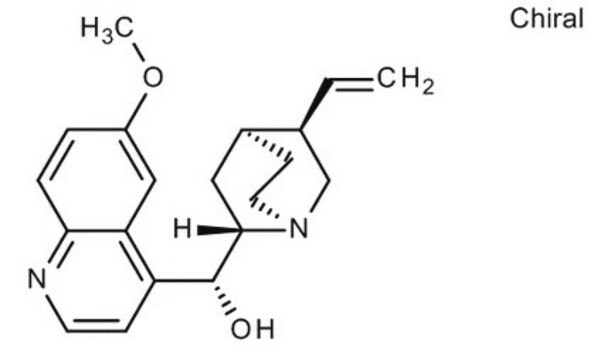
Chaîne SMILES
COc1ccc2nccc([C@@H](O)[C@@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1
InChI
1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19-,20+/m0/s1
Clé InChI
LOUPRKONTZGTKE-WZBLMQSHSA-N
Informations sur le gène
human ... ABCB1(5243) , CYP2C9(1559) , CYP2D6(1565)
rat ... Cyp2d1(266684) , Cyp2d2(25053) , Cyp2d3(24303) , Cyp2d4v1(171522)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- To study its in vitro antimalarial activity in combination with omeprazole.
- To analyze its effect on viscosity and friction of saliva.
- As a test agent to study its impact on the accumulation of the fluorescent P-glycoprotein (Pgp) substrates in P-glycoprotein overexpressing breast cancer cells.
- To study its influence on the pyramidal cell intrinsic properties, extracellular potassium transients, and epileptiform activity in vitro.
- As a reference compound to identify alkaloids by phytochemical screening of Deianira erubescens, Strychnos pseudoquina and Remijia ferruginea plants.
Actions biochimiques/physiologiques
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Skin Sens. 1
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 1
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique