Alle Fotos(1)
Wichtige Dokumente
H6891
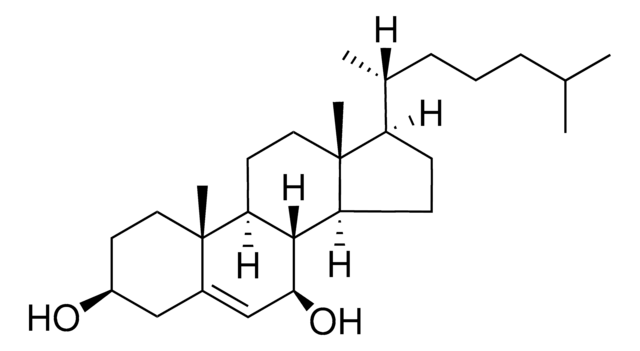
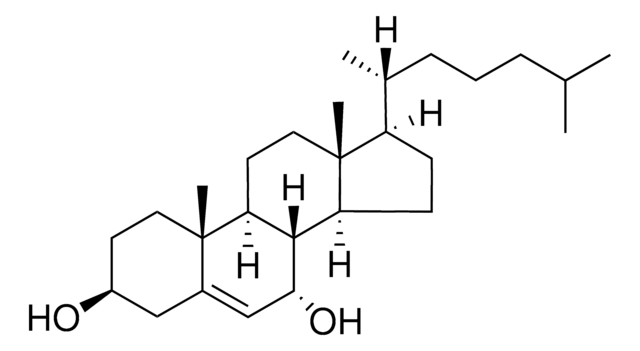
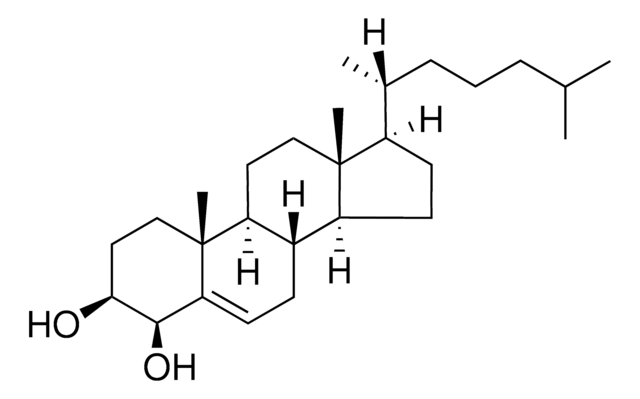
7β-Hydroxycholesterol
≥95%
Synonym(e):
5-Cholestene-3β,7β-diol
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C27H46O2
CAS-Nummer:
Molekulargewicht:
402.65
MDL-Nummer:
UNSPSC-Code:
12352211
PubChem Substanz-ID:
NACRES:
NA.77
Empfohlene Produkte
Qualitätsniveau
Assay
≥95%
Form
powder
Funktionelle Gruppe
hydroxyl
Versandbedingung
ambient
Lagertemp.
room temp
SMILES String
[H][C@@]12[C@]([C@](CC[C@H](O)C3)(C)C3=C[C@@H]2O)([H])CC[C@@]4(C)[C@@]1([H])CC[C@]4([H])[C@]([H])(C)CCCC(C)C
InChI
1S/C27H46O2/c1-17(2)7-6-8-18(3)21-9-10-22-25-23(12-14-27(21,22)5)26(4)13-11-20(28)15-19(26)16-24(25)29/h16-18,20-25,28-29H,6-15H2,1-5H3
InChIKey
OYXZMSRRJOYLLO-UHFFFAOYSA-N
Anwendung
7β-hydroxycholesterol was used to study oxysterol-induced apoptosis in human endothelial cells.
Biochem./physiol. Wirkung
7β-Hydroxycholesterol is an oxysterol, the enzymatic or non-enzymatic product of cholesterol oxidation. Oxysterols are cytotoxic and induce death in monocytes, smooth muscle cells and endothelial cells. The mechanism of apoptosis induced by oxysterols may involve caspases or DNA fragmentation. Increased levels of 7β-Hydroxycholesterol correlates with increased risk of cardiovascular diseases including atherosclerosis.
Angaben zur Herstellung
7β-Hydroxycholesterol yields clear, colorless to faint yellow solution in ethanol, with or without heating.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
G Lizard et al.
FEBS letters, 419(2-3), 276-280 (1998-01-15)
The oxysterols, 7beta-hydroxycholesterol and 7-ketocholesterol, are involved in the cytotoxicity of oxidized LDL. To elucidate their molecular mechanisms, the human promonocytic leukemia cells U937 and U4 were used. U4 cells overexpressing Bcl-2 were obtained by transfection of U937 cells. 7Beta-hydroxycholesterol
Raymond C S Seet et al.
Free radical research, 47(4), 283-290 (2013-01-25)
The purpose of this study was to evaluate the use of Framingham risk scores (FRRs) to identify high-risk individuals with biochemical evidence of increased oxidative damage, who may benefit from antioxidant therapies. A bimodal change in plasma F2-isoprostane levels was
Ludovic Clarion et al.
Biochemical pharmacology, 83(1), 37-46 (2011-10-11)
7β-Hydroxycholesterol cytotoxicity has been shown in vivo and in vitro to be dependent on the accumulation of its esters. We show in our study, using a detergent-free raft preparation and LC/MS lipid content analysis, that membrane microdomains isolated from 7β-hydroxycholesterol-treated
B Ziedén et al.
Arteriosclerosis, thrombosis, and vascular biology, 19(4), 967-971 (1999-04-09)
The mortality in coronary heart disease among 50- to 54-year-old men is 4 times higher in Lithuania than in Sweden. It was recently suggested that traditional risk factors could not explain this mortality difference. LDL of Lithuanian men showed, however
Paola Gamba et al.
Aging cell, 10(3), 403-417 (2011-01-29)
All three cholesterol oxidation products implicated thus far in the pathogenesis of Alzheimer's disease, 7β-hydroxycholesterol, 24-hydroxycholesterol, and 27-hydroxycholesterol, markedly enhance the binding of amyloid-beta (Aβ) to human differentiated neuronal cell lines (SK-N-BE and NT-2) by up-regulating net expression and synthesis
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.