W506907
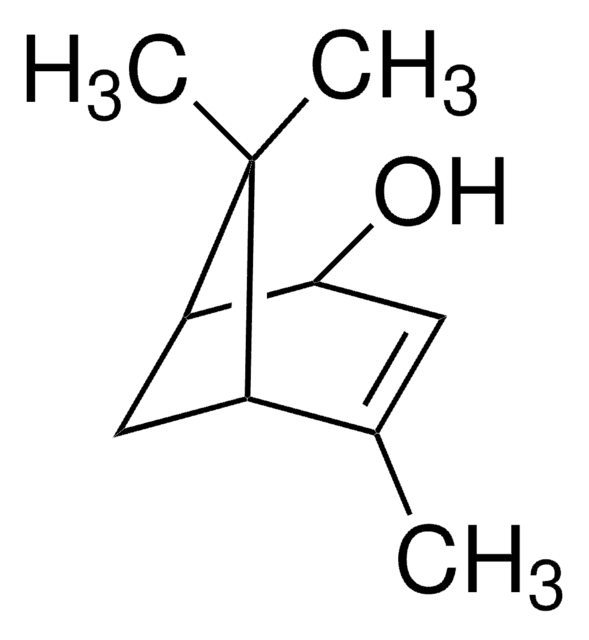
(1S)-(−)-Verbenon
≥93%
Synonym(e):
(1S,5S)-2-Pinen-4-on, (1S,5S)-4,6,6-Trimethylbicyclo[3.1.1]hept-3-en-2-on
About This Item
Empfohlene Produkte
Biologische Quelle
synthetic
Qualitätsniveau
Qualität
Fragrance grade
Halal
Kosher
Agentur
follows IFRA guidelines
Einhaltung gesetzlicher Vorschriften
EU Regulation 1223/2009
Assay
≥93%
Optische Aktivität
[α]20/D −140°, c = 10 in ethanol
Brechungsindex
n20/D 1.496 (lit.)
bp
227-228 °C (lit.)
Dichte
0.975 g/mL at 20 °C (lit.)
Anwendung(en)
flavors and fragrances
Dokumentation
see Safety & Documentation for available documents
Nahrungsmittelallergen
no known allergens
Allergener Duftstoff
no known allergens
Organoleptisch
camphoraceous; minty; spicy
SMILES String
CC1=CC(=O)[C@H]2C[C@@H]1C2(C)C
InChI
1S/C10H14O/c1-6-4-9(11)8-5-7(6)10(8,2)3/h4,7-8H,5H2,1-3H3/t7-,8+/m0/s1
InChIKey
DCSCXTJOXBUFGB-JGVFFNPUSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Anwendung
- Anti-Inflammatory and Antinociceptive Activities of the Essential Oil of Tagetes parryi A. Gray (Asteraceae) and Verbenone.: This article reports on the anti-inflammatory and pain-relief effects of (1S)-(−)-Verbenone as part of the essential oil of Tagetes parryi, suggesting potential therapeutic applications for pain management and inflammation (González-Velasco et al., 2022).
Haftungsausschluss
Anwendung
Lagerklassenschlüssel
10 - Combustible liquids
WGK
WGK 3
Flammpunkt (°F)
185.0 °F - closed cup
Flammpunkt (°C)
85 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Chromatograms
suitable for GCsuitable for GCUnser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.
![Bicyclo[2.2.1]hept-2-en 99%](/deepweb/assets/sigmaaldrich/product/structures/270/492/95fd4909-6108-4858-8c94-609b54387149/640/95fd4909-6108-4858-8c94-609b54387149.png)