Wichtige Dokumente
W266418
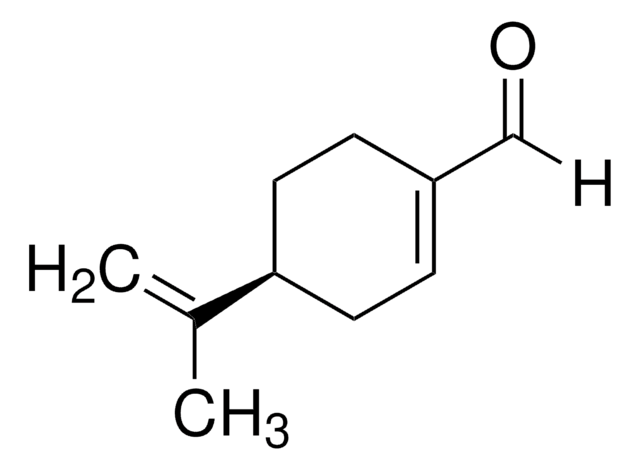
(S)-(−)-Perillylalkohol
≥95%, FG
Synonym(e):
p-Mentha-1,8-dien-7-ol
About This Item
Empfohlene Produkte
Biologische Quelle
synthetic
Qualitätsniveau
Qualität
FG
Halal
Kosher
Einhaltung gesetzlicher Vorschriften
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 117
FDA 21 CFR 172.515
Assay
≥95%
Optische Aktivität
[α]20/D −88°, c = 1 in methanol
Brechungsindex
n20/D 1.501 (lit.)
bp
119-121 °C/11 mmHg (lit.)
Dichte
0.96 g/mL at 25 °C (lit.)
Anwendung(en)
flavors and fragrances
Dokumentation
see Safety & Documentation for available documents
Nahrungsmittelallergen
no known allergens
Organoleptisch
fatty; green
SMILES String
CC(=C)[C@H]1CCC(CO)=CC1
InChI
1S/C10H16O/c1-8(2)10-5-3-9(7-11)4-6-10/h3,10-11H,1,4-7H2,2H3/t10-/m1/s1
InChIKey
NDTYTMIUWGWIMO-SNVBAGLBSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Anwendung
- CYP108N12 initiates p-cymene biodegradation in Rhodococcus globerulus.: This study explores the enzymatic breakdown pathways of monoterpenes, using (S)-(−)-Perillyl alcohol as a precursor, offering insights into microbial degradation processes that could be vital for bioremediation efforts or synthetic biology applications (Giang et al., 2022).
- Orofacial antinociceptive effects of perillyl alcohol associated with codeine and its possible modes of action.: Research demonstrates the pain-relieving properties of (S)-(−)-Perillyl alcohol when combined with codeine, highlighting its potential for developing new analgesic formulations in dental and facial pain management (Limeira et al., 2022).
- Orofacial antinociceptive activity of (S)-(-)-perillyl alcohol in mice: a randomized, controlled and triple-blind study.: This study underpins the effectiveness of (S)-(−)-Perillyl alcohol in reducing orofacial pain in a controlled experimental setup, providing a basis for further clinical trials in pain management (Tomaz-Morais et al., 2017).
- In Vivo Anti-Tumor Activity and Toxicological Evaluations of Perillaldehyde 8,9-Epoxide, a Derivative of Perillyl Alcohol.: Highlights the anti-tumor properties of a novel derivative of (S)-(−)-Perillyl alcohol, suggesting its potential as a therapeutic agent in oncology, with comprehensive studies on its efficacy and safety (Andrade et al., 2016).
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
10 - Combustible liquids
WGK
WGK 3
Flammpunkt (°F)
230.0 °F - closed cup
Flammpunkt (°C)
110 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| W266418-1KG | |
| W266418-1KG-K | 4061837800429 |
| W266418-5KG | |
| W266418-100G | |
| W266418-100G-K | 4061837800412 |
| W266418-5KG-K | 4061836712150 |
| W266418-SAMPLE | |
| W266418-SAMPLE-K | 4061837800436 |
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.