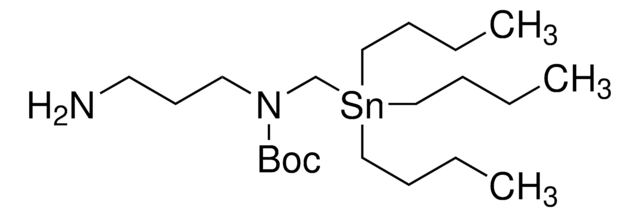
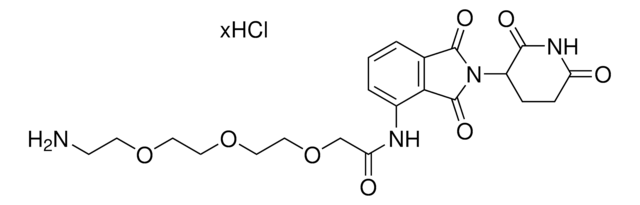
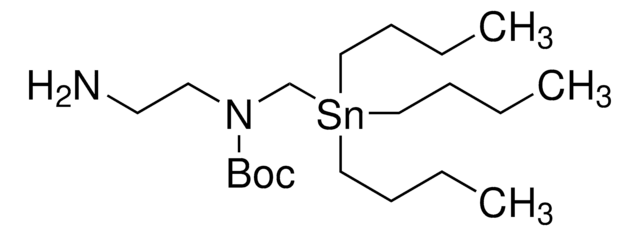
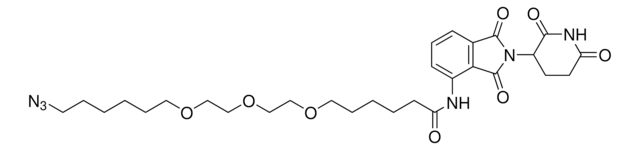
900401
SLAP 2,3-Bicyclo-(3,4-THF) N-BnPip Reagent
≥95%
Synonym(e):
trans-N3-Benzyl-N3-((trimethylsilyl)methyl)tetrahydrofuran-3,4-diamine
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
≥95%
Form
liquid
Brechungsindex
n/D 1.520
Dichte
1.000 g/mL
Funktionelle Gruppe
amine
ether
phenyl
Lagertemp.
−20°C
Verwandte Kategorien
Anwendung
Sonstige Hinweise
- Technology Spotlight: SLAP Reagents for Piperazine Synthesis
- Silicon Amine Reagents for the Photocatalytic Synthesis of Piperazines from Aldehydes and Ketones
- Lewis Acid Induced Toggle from Ir(II) to Ir(IV) Pathways in Photocatalytic Reactions: Synthesis of Thiomorpholines and Thiazepanes from Aldehydes and SLAP Reagents.
- Continuous Flow Synthesis of Morpholines and Oxazepanes with Silicon Amine Protocol (SLAP) Reagents and Lewis Acid Facilitated Photoredox Catalysis
Ähnliches Produkt
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
10 - Combustible liquids
WGK
WGK 3
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Die passende Version wird nicht angezeigt?
Wenn Sie eine bestimmte Version benötigen, können Sie anhand der Lot- oder Chargennummer nach einem spezifischen Zertifikat suchen.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Protokolle
The Bode group has developed SnAP (stannyl amine protocol) reagents that cross-couple with aldehydes and ketones to provide one-step access to a wide variety of saturated N-heterocycles.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.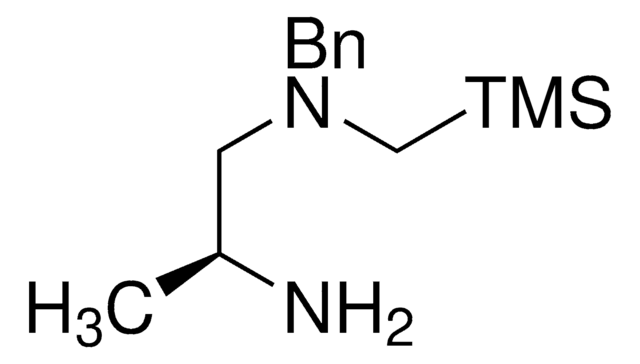
![4-((Trimethylsilyl)ethynyl)-1H-pyrrolo[2,3-b]pyridine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/967/461/0ea68fc7-b577-45e4-a273-df90243ff9ab/640/0ea68fc7-b577-45e4-a273-df90243ff9ab.png)
![5-(Trifluoromethyl)-4-(trimethylsilyl)-1H-pyrrolo[2,3-b]pyridine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/171/524/fda192b0-357a-4bd7-85e1-475ee8ce5b2c/640/fda192b0-357a-4bd7-85e1-475ee8ce5b2c.png)