The Endothelial Cell Transwell Migration and Invasion Assay
Angiogenesis is a tightly regulated cellular event that is balanced by pro- and antiangiogenic signals including integrins, chemokines, angiopoietins, oxygen sensing agents, junctional molecules and endogenous inhibitors.4 While physiological angiogenesis is highly organized, pathological angiogenesis is less well controlled, with vessels rarely maturing, remodeling or regressing in response to disease.5 Since endothelial cell migration and invasion are essential to angiogenesis, the so-called transwell cell migration and invasion assays are helpful tools for studying the underlying mechanisms of angiogenic events. Cell migration can simply be defined as the process by which cells move from one location to the other directed by extrinsic biochemical signals. Cell invasion, on the other hand, measures the ability of endothelial cells to move through a 3D matrix (such as basement membranes). It is a complex multistep process that involves adhesion, proteolysis of ECM components, reorganization of the microenvironment, and migration through the matrix.6
The in vitro transmigration assay, also known as the Boyden chamber assay, is used to measure the migration of endothelial cells along a cytokine gradient (chemotaxis) and to measure the chemotactic capability of test substances in vitro. It involves two medium-filled compartments separated by a microporous membrane. As a rule, cells are placed in the upper compartment and allowed to migrate through the pores of the membrane into the lower compartment, where chemotactic agents are present. After an appropriate incubation time, the membrane between the two compartments is fixed and the number of cells that have migrated to the lower side is determined.
The in vitro invasion assay measures cell migration of endotheial cells through an extracellular matrix. Invasive migration is a major process in angiogenesis but also plays a significant role in pathological events such as cancer development and metastasis.9 Like the transwell migration assay, the cell invasion assay involves two compartments separated by a membrane with a precisely defined pore size. The membrane is covered by a matrix that mimics the basement membrane of blood vessels. A potential chemotactic factor is placed in one compartment and a gradient develops across the membrane. Endothelial cells introduced to the other compartment degrade the matrix and then migrate along this gradient. After a suitable incubation time, the cells that have migrated through the membrane can be counted after fixing the membrane and staining them.9

Figure 1.Workflow of endothelial cell transmigration and invasion assays.
Endothelial Cell Transwell Migration and Invasion Assay Protocols
Transmigration Assay Protocol
1. Prepare a culture of endothelial cells. Plate PromoCell endothelial cells at 5X103 cells/cm2 (or as recommended in the respective product manual) in a suitable culture vessel using the recommended endothelial growth medium. Replace culture medium every 2-3 days. Allow the cells to reach 70-90% confluency.
2. Prepare the assay medium. Prepare an appropriate amount of assay medium by adding 10% FBS to the Endothelial Cell Basal Medium (e.g. 9 mL basal medium + 1 mL FBS).
3. Adjust media and reagents to room temperature. Pre-warm the assay medium and the components of the PromoCell DetachKit at room temperature for 1-2 hours.
4. Prepare the test medium. Dissolve the test substance in assay medium. 750 μL of the test medium should be used per well of the 24-well plate. We recommend to prepare one additional test medium with 20 ng/mL VEGF or bFGF to be used as a positive control. As negative control assay medium without any chemotactic factor can be used.
5. Prepare 24-well plates and add test medium. Place cell culture inserts into the wells of the 24-well plate. Add 750 μL test medium to the outer compartment of each well.
6. Detach endothelial cells. Endothelial cells should be 70-90% confluent. Remove medium from the culture vessel and wash the cells by adding 200 μL/cm2 PBS (D8537). Remove the PBS and add 100 μL/cm2 Trypsin/EDTA (T3924). Close the vessel and examine cells under a microscope. When the cells start to detach, gently tap the side of the vessel to loosen the remaining cells. Neutralize trypsin solution by adding 100 μL/cm2 Trypsin Neutralization Solution (T6414) and gently shake the culture vessel for 30 seconds.
7. Count the cells. Transfer the cell suspension into a centrifuge tube and spin the tube at 220 x g for 4 min at room temperature. Remove the supernatant and resuspend the cell pellet in 5 mL assay medium. Determine cell number according to your standard procedure.
8. Seed the cells in the cell culture inserts. Dilute the cell concentration to 1x 105 cells/mL with assay medium. Carefully add 500 μL of the cell suspension (= 5X104 cells) into the cell culture inserts in the 24-well plate.
9. Start transmigration experiment. Incubate cells in the 24-well plate inserts in a humidified incubator (37 °C, 5% CO2) for 3-6 hours. During the incubation time a gradient of the test substance develops across the membrane. Cells migrate through pores along the gradient to the bottom side of the membrane and adhere there.
10. Remove non-migrated cells on the upper side of the membrane. Carefully remove the medium from all cell culture inserts. Then, remove the inserts using tweezers and aspirate the medium from each well. After returning the inserts into the well, remove non-migrated cells on the upper side of the membrane using a cotton swab. Pipet PBS into the gap between the well and the insert.
11. Proceed with fixation and staining of migrated cells.
Invasion Assay Protocol
1. Prepare a culture of endothelial cells. Plate PromoCell Endothelial Cells at 5X103 cells/cm2 in a suitable culture vessel using the recommended endothelial growth medium. Replace culture medium every 2-3 days. Allow the cell to reach 70-90% confluency.
2. Prepare invasion assay chambers and adjust media and reagents to appropriate temperatures. Prepare an appropriate amount of assay medium by adding 10% FBS to the Endothelial Cell Basal Medium (e.g. 9 mL basal medium + 1 mL FBS). Add 750 μL assay medium to each well and 500 μL of Matrigel/ECM Gel to the cell culture insert. Place the 24-well plate in a humidified incubator (37 °C, 5% CO2) for 1-2 hours.
3. Prepare the test medium. Dissolve the test substance in assay medium. 750 μL of the test medium should be used per well of the 24-well plate. We recommend to prepare one additional test medium with 20 ng/mL VEGF or bFGF to be used as a positive control. As negative control assay medium without any chemotactic factor can be used.
4. Add the test medium to the wells. Remove the Matrigel/ECM Gel coated inserts from the wells of the 24-well plate using tweezers and aspirate the assay medium from each well. After returning the inserts into the wells, pipet 750 μL test medium into the gap between the well and the insert.
5. Detach endothelial cells. Endothelial cells should be 70-90% confluent. Remove medium from the culture vessel and wash the cells by adding 200 μL/cm2 PBS (D8537). Remove the PBS and add 100 μL/cm2 Trypsin/EDTA (T3924). Close the vessel and examine cells under a microscope. When the cells start to detach, gently tap the side of the vessel to loosen the remaining cells. Neutralize trypsin solution by adding 100 μL/cm2 Trypsin Neutralization Solution (T6414) and gently shake the culture vessel for 30 seconds.
6. Count the cells. Transfer the cell suspension into a centrifuge tube and spin the tube at 220 x g for 4 min at room temperature. Remove the supernatant and resuspend the cell pellet in 5 mL assay medium. Determine cell number according to your standard procedure.
7. Seed the cells in the Matrigel/ECM Gel coated cell culture inserts. Dilute the cell concentration to 1x105 cells/mL with assay medium. Remove the assay medium from the Matrigel-coated inserts without touching the Matrigel coated surface. Carefully add 500 μL of the cell suspension (= 5X104 cells) into the cell culture inserts.
8. Start invasion experiment. Incubate cells in the Matrigel-coated inserts in a humidified incubator (37 °C, 5% CO2) for 16-28 hours. During the incubation time a gradient of the test substance develops across the membrane. In case of a positive stimulation by the test substance cells degrade the matrix, migrate through pores along the gradient to the bottom side of the membrane and adhere there.
9. Remove non-migrated cells on the upper side of the membrane. Carefully remove the medium from all Matrigel/ECM Gel coated inserts. Then, remove the inserts using tweezers and aspirate the medium from each well. After returning the inserts into the well, remove non-migrated cells on the upper side of the membrane using a cotton swab. Pipet 750 μL PBS into the gap between the well and the insert.
10. Proceed with Fixation and Staining of migrated cells.
Analysis of Migrated Cells
Cell Fixation and Staining
1. Fix the membranes and dry the inserts. Carefully remove the PBS from the wells and add 750 μL cold methanol (34860) (4 °C) to the wells. Incubate for 20 min at room temperature. Carefully remove the methanol and wait for 30 minutes. During this time the membrane will air dry. After drying, the membrane can be stored at 4 °C and processed later.
2. Stain the cells. Add 750 μL Crystal Violet (V5265) to the wells and incubate for 20 minutes at room temperature.
3. Wash the membrane. Carefully remove the staining solution and wash three times thoroughly with distilled water.
4. Proceed with analysis.
Analysis
1. Visual quantification. Fill 750 μL distilled water in the wells of the 24-well plate and count the migrated cells on the lower site of the membrane using an inverted microscope.
2. Colorimetric quantification with a microplate reader. Fill 750 μL 10% acetic acid (A6283) in the wells and incubate for 30 seconds while carefully shaking the 24-well plate. The cells on the membrane will be lysed by the acetic acid and the Crystal Violet in the cells will be released. Remove the insert from the 24-well plate and read the optical density of the 10% acetic acid at 595 nm using a microplate reader.
Results
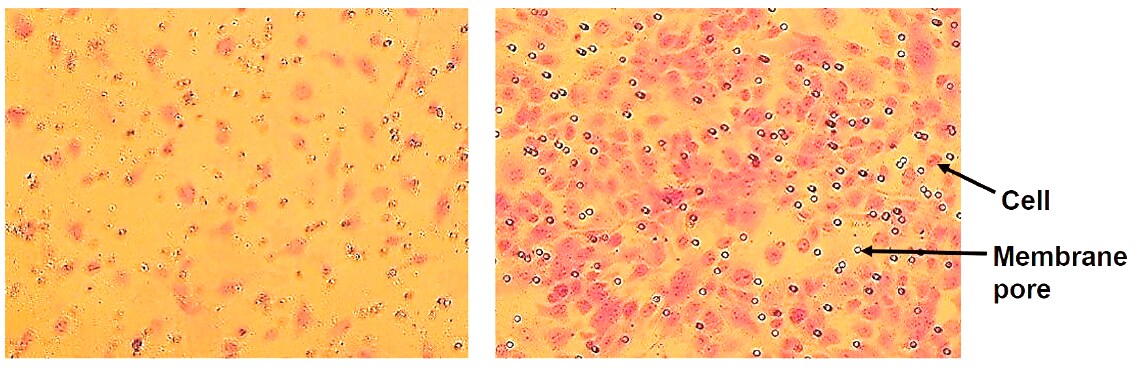
Figure 2.In vitro cell migration of endothelial cells stimulated by VEGF. Cells that have migrated through the membrane without VEGF (left) and with 20 ng/ml VEGF in the medium (right). Cells are stained with Crystal Violet.
References
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?