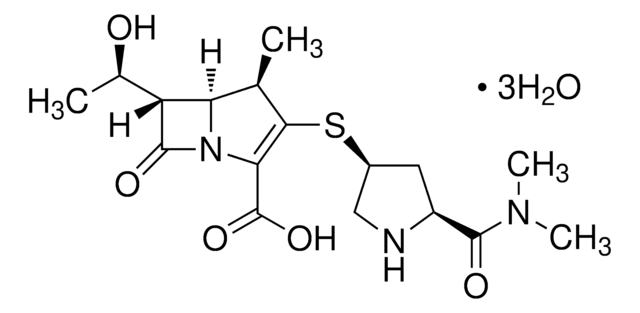
PHR1654
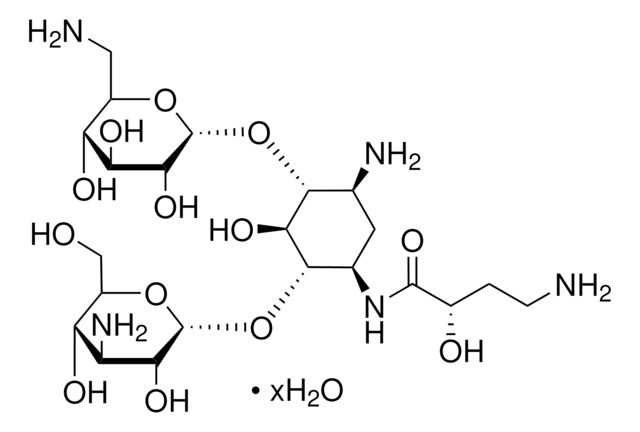
Amikacin
Pharmaceutical Secondary Standard; Certified Reference Material
Sinónimos:
Amikacin
About This Item
Productos recomendados
grade
certified reference material
pharmaceutical secondary standard
Quality Level
agency
traceable to Ph. Eur.
traceable to USP 1019508
API family
amikacin
CofA
current certificate can be downloaded
packaging
pkg of 1 g
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-8°C
SMILES string
N([C@H]1[C@@H]([C@H]([C@@H]([C@H](C1)N)O[C@H]3O[C@@H]([C@H]([C@@H]([C@H]3O)O)O)CN)O)O[C@H]2O[C@@H]([C@H]([C@@H]([C@H]2O)N)O)CO)C(=O)[C@@H](O)CCN
InChI
1S/C22H43N5O13/c23-2-1-8(29)20(36)27-7-3-6(25)18(39-22-16(34)15(33)13(31)9(4-24)37-22)17(35)19(7)40-21-14(32)11(26)12(30)10(5-28)38-21/h6-19,21-22,28-35H,1-5,23-26H2,(H,27,36)/t6-,7+,8-,9+,10+,11-,12+,13+,14+,15-,16+,17-,18+,19-,21+,22+/m0/s1
InChI key
LKCWBDHBTVXHDL-RMDFUYIESA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
Analysis Note
Other Notes
Footnote
related product
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico