E3876
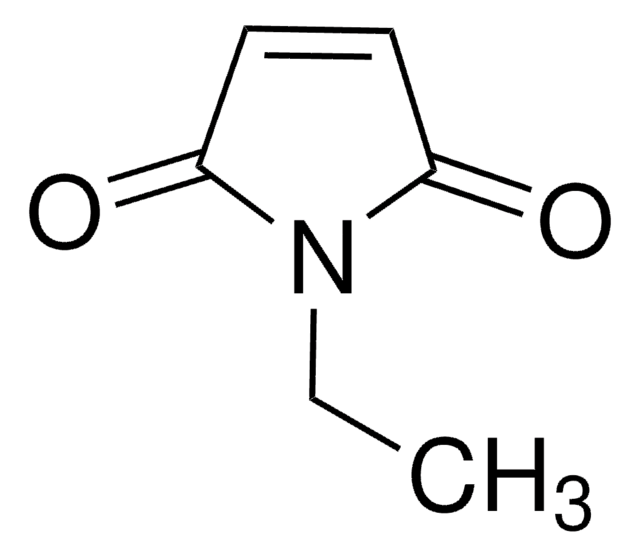
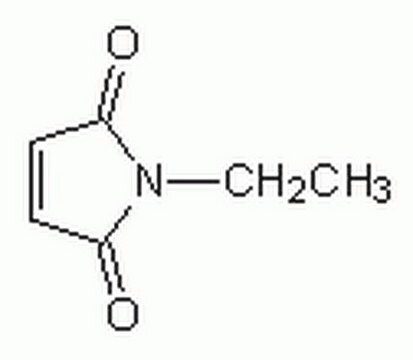
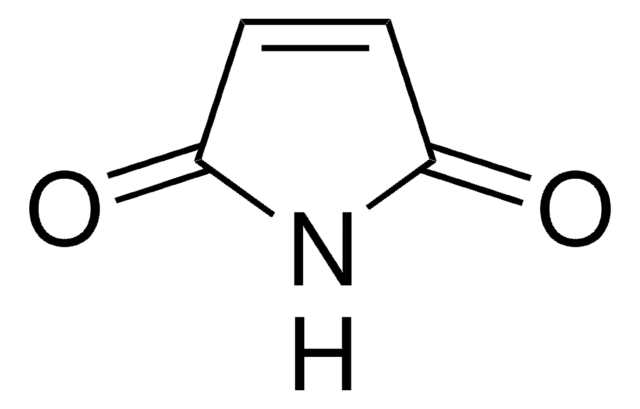
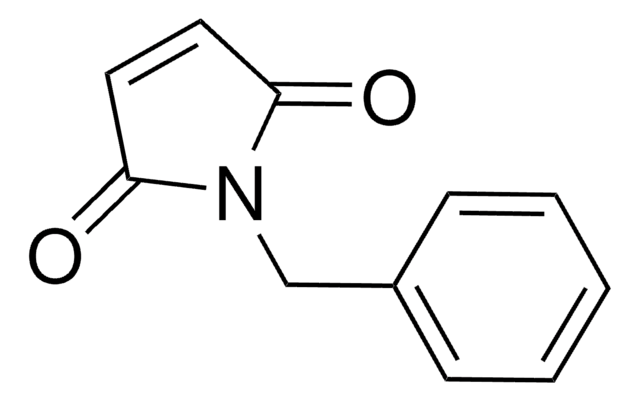
N-Ethylmaleimide
crystalline, ≥98% (HPLC)
Sinónimos:
NEM
About This Item
Productos recomendados
biological source
synthetic (organic)
Quality Level
assay
≥98% (HPLC)
form
crystalline
storage condition
(Tightly closed. Dry. Keep in a well-ventilated place. )
technique(s)
activity assay: suitable
co-immunoprecipitation (co-IP): suitable
immunoblotting: suitable
color
white to faint yellow
bp
210 °C (lit.)
mp
43-46 °C (lit.)
solubility
ethanol: 50 mg/mL, clear to slightly hazy, colorless to faint yellow or tan
suitability
suitable for Western blot
application(s)
life science and biopharma
storage temp.
2-8°C
SMILES string
CCN1C(=O)C=CC1=O
InChI
1S/C6H7NO2/c1-2-7-5(8)3-4-6(7)9/h3-4H,2H2,1H3
InChI key
HDFGOPSGAURCEO-UHFFFAOYSA-N
Gene Information
human ... KCNQ2(3785) , KCNQ4(9132) , KCNQ5(56479) , SLC6A3(6531) , SLC6A4(6532)
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
Biochem/physiol Actions
Preparation Note
Other Notes
comparable product
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 2 Oral - Acute Tox. 3 Dermal - Eye Dam. 1 - Skin Corr. 1B - Skin Sens. 1
Storage Class
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 3
flash_point_f
163.2 °F - closed cup
flash_point_c
72.9 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico