8.51006
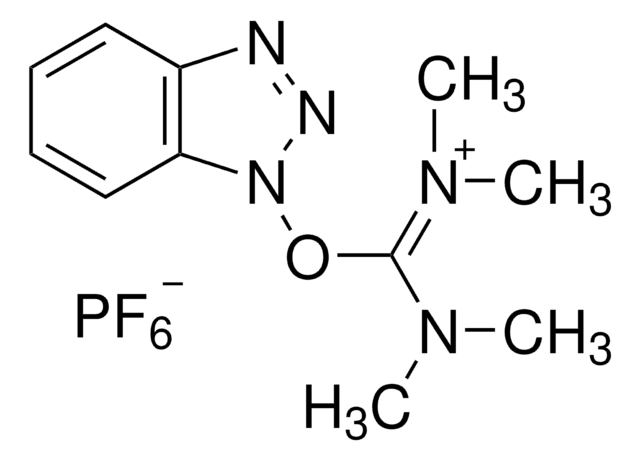
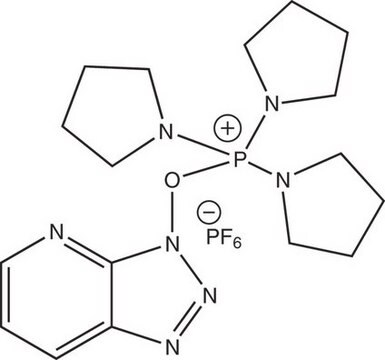
HBTU
≥99.0% (HPLC), for peptide synthesis, Novabiochem®
Sinónimos:
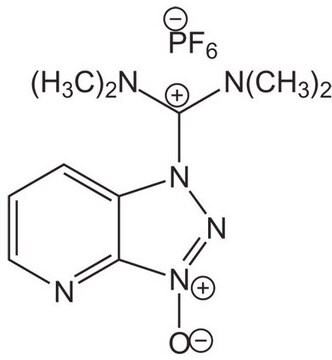
HBTU, 2-(1H-Benzotriazole-1-yl)-1,1,3,3-tetramethylaminium hexafluorophosphate
About This Item
Productos recomendados
product name
HBTU, 2-(1H-Benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate Novabiochem®
Quality Level
product line
Novabiochem®
assay
≥99.0% (HPLC)
form
powder
potency
>2000 mg/kg LD50, oral (Rat)
reaction suitability
reaction type: Coupling Reactions
manufacturer/tradename
Novabiochem®
pH
4.1 (1.6 g/L in H2O)
mp
250 °C
solubility
1.6 g/L
application(s)
peptide synthesis
storage temp.
2-8°C
InChI
1S/C11H16N5O/c1-14(2)11(15(3)4)17-16-10-8-6-5-7-9(10)12-13-16/h5-8H,1-4H3/q+1
InChI key
CLZISMQKJZCZDN-UHFFFAOYSA-N
General description
Associated Protocols and Technical Articles
Guide to Selection of Coupling Reagents
Literature references
[1] R. Knorr, et al. (1989) Tetrahedron Lett., 30, 1927.
[2] M. S. Bernatowicz, et al. (1989) Tetrahedron Lett., 30, 4645.
[3] D. Ambrosius, et al. (1989) Biol. Chem. Hoppe-Seyler, 370, 217.
[4] C. G. Fields, et al. (1991) Pept. Res., 4, 95.
[5] A. G. Beck-Sickinger, et al. (1991) Pept. Res., 4, 88.
[6] G. E. Reid, et al. (1992) Anal. Biochem., 200, 301.
[7] G. B. Fields, et al. in ′Innovation & Perspectives in Solid Phase Synthesis, 1st International Symposium′, R. Epton (Eds), SPCC UK Ltd., Birmingham, 1990, pp. 241.
[8] P. A. Baybayan, et al. in ′Peptides, Chemistry & Biology, Proc. 12th American Peptide Symposium′, J. A. Smith & J. E. Rivier (Eds), ESCOM, Leiden, 1992, pp. 566.
[9] J. J. Dudash, et al. (1993) Synth. Commun., 23, 349.
Application
- Synthesis of Quinoxaline Derivatives Using HBTU: A study highlighting the use of HBTU as a Lewis acid catalyst for synthesizing quinoxaline derivatives, presenting a mild and green protocol (Popatkar and Meshram, 2020).
- Efficient Conversion of Carboxylic Acids into Benzimidazoles: Details an HBTU-promoted methodology for converting carboxylic acids into benzimidazoles in a one-pot strategy (Barasa and Yoganathan, 2018).
- Synthesis of Malonyl-linked Glycoconjugates: Discusses the use of HBTU in the synthesis of glycoconjugates, comparing its efficiency with other reagents (Nörrlinger et al., 2016).
Linkage
Analysis Note
Appearance of substance (visual): powder
Identity (IR): passes test
Assay (HPLC, area%): ≥ 99.0 % (a/a)
Solubility (1 mmole in 2 ml DMF): clearly soluble
Legal Information
signalword
Warning
hcodes
Hazard Classifications
Skin Sens. 1A
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Novabiochem® offers a large number of coupling reagents for in situ activation. In situ activating reagents are easy to use, fast reacting – even with sterically hindered amino acids, and their use is generally free of side reactions.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico