445460
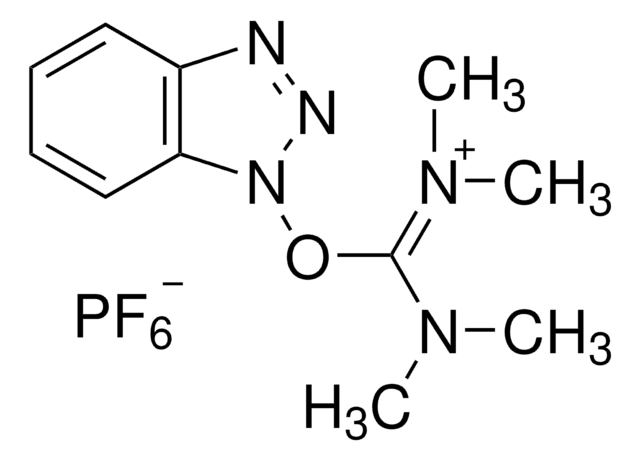
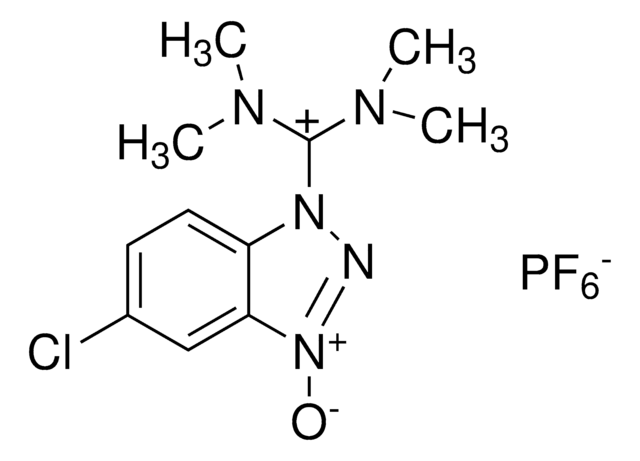
HATU
97%, for peptide synthesis
Sinónimos:
1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxid hexafluorophosphate, N-[(Dimethylamino)-1H-1,2,3-triazolo-[4,5-b]pyridin-1-ylmethylene]-N-methylmethanaminium hexafluorophosphate N-oxide
About This Item
Productos recomendados
product name
HATU, 97%
Quality Level
assay
97%
form
solid
reaction suitability
reaction type: Coupling Reactions
mp
183-185 °C (lit.)
application(s)
peptide synthesis
functional group
amine
storage temp.
2-8°C
SMILES string
F[P-](F)(F)(F)(F)F.CN(C)[C+](N(C)C)n1n[n+]([O-])c2ncccc12
InChI
1S/C10H15N6O.F6P/c1-13(2)10(14(3)4)15-8-6-5-7-11-9(8)16(17)12-15;1-7(2,3,4,5)6/h5-7H,1-4H3;/q+1;-1
InChI key
FKBFHOSFPRWJNV-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
Synthesis of Aurora A kinase inhibitors
HPLC assay to determine D- and L- acid enantiomers in human plasma
Amide bond formation reactions
Catalyst for:
Selective acylation
Selecocyclization-oxidation deselenation sequence
signalword
Danger
hcodes
Hazard Classifications
Resp. Sens. 1 - Skin Sens. 1A
supp_hazards
Storage Class
4.1A - Other explosive hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
The special need of SPPS for rapid and highly efficient coupling reagents led to the development of several new reagents starting from BOP (Benzotriazol-1-yloxytris(dimethylamino)phosphonium hexafluorophosphate).
COMU is a non-explosive coupling agent suitable for solution phase & solid phase peptide synthesis. Its activity meets or exceeds that of HATU and its water-soluble by-product are easily removed.
Amide bonds are ubiquitous in both nature and industrial applications. They are vital to the structure and function of biological macromolecules and polymers. The importance of this functionality has resulted in numerous approaches to its formation, ranging from stoichiometric activation of carboxylic acids to more recent advances in catalytic amide bond formation.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico