697400
3-Hexylthiophene-2-boronic acid pinacol ester
95%
Sinónimos:
2-(3-Hexyl-2-thienyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
About This Item
Productos recomendados
Quality Level
assay
95%
form
liquid
refractive index
n20/D 1.490-1.499
density
0.983 g/mL at 25 °C
SMILES string
CCCCCCc1ccsc1B2OC(C)(C)C(C)(C)O2
InChI
1S/C16H27BO2S/c1-6-7-8-9-10-13-11-12-20-14(13)17-18-15(2,3)16(4,5)19-17/h11-12H,6-10H2,1-5H3
InChI key
XCXAUPBHQCCWCI-UHFFFAOYSA-N
Application
- Suzuki-Miyaura cross-coupling reactions
- p-type/n-type switching of ambipolar bithiazole-benzothiadiazole-based polymers in solar cells
- Hierarchical self-assembly of semiconductor functionalized peptide a-helixes and optoelectronic properties
Reagent used in Preparation of
- Photovoltaic materials, polymers, and thiophene-based compounds with photophysical, electrochemical, and fluorescent properties
- Polymer solar cells for Low band gap poly(1,4-arylene-2,5-thienylene)s with benzothiadiazole units
- Dithienothiophene-based dyes for dye-sensitized solar cells
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
>230.0 °F
flash_point_c
> 110 °C
ppe
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
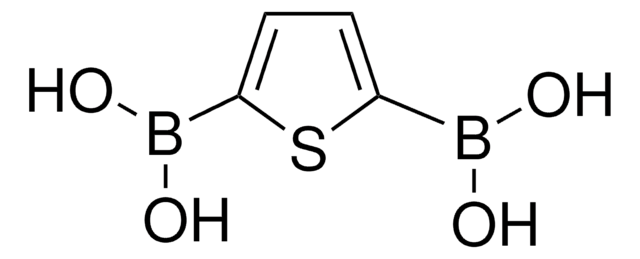
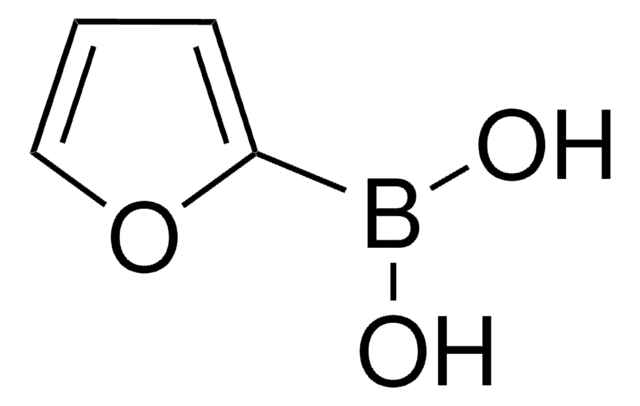
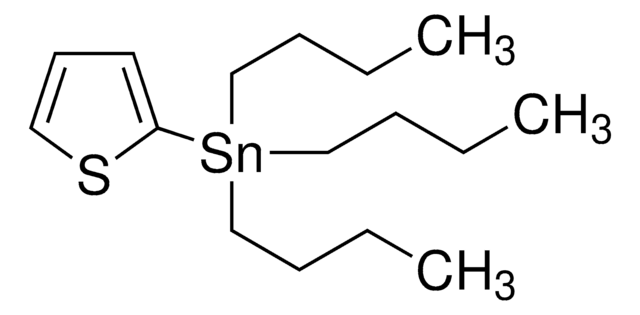
Los clientes también vieron
Artículos
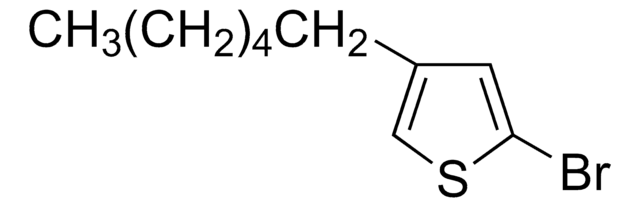
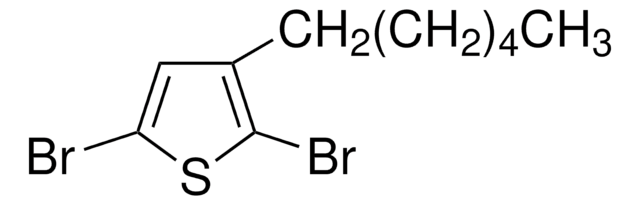
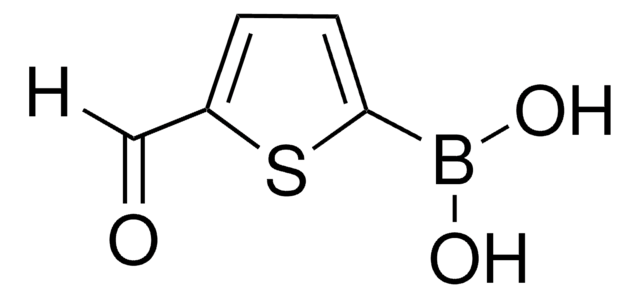
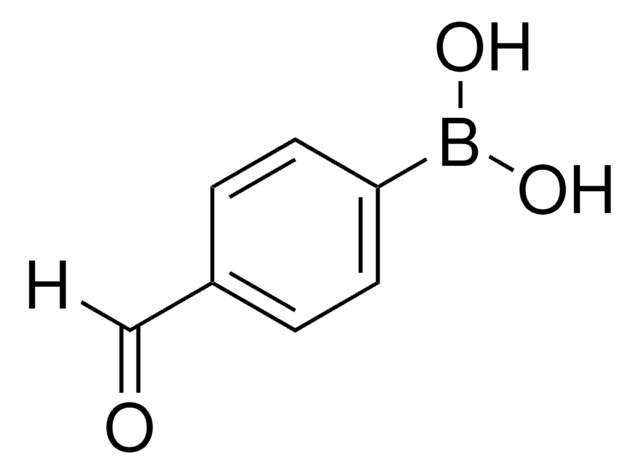
Oligothiophenes are important organic electronic materials which can be produced using synthetic intermediates and Suzuki coupling.
The synthesis of biaryl compounds via the Suzuki–Miyaura coupling reaction has become more commonplace now that many arylboronic acids are readily available.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico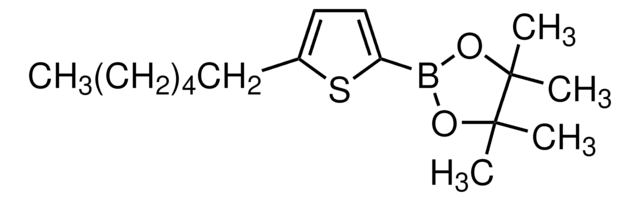



![4,7-Dibromobenzo[c][1,2,5]thiadiazole 95%](/deepweb/assets/sigmaaldrich/product/structures/711/964/3fd3ffd1-5916-468e-a743-22f1611b5a33/640/3fd3ffd1-5916-468e-a743-22f1611b5a33.png)