Solid Phase Synthesis
Peptides are manufactured using solid phase FMOC or BOC chemistry methodologies on a PEG-Polystyrene support resin. Upon synthesis completion, side chain protecting groups are removed and the peptides are simultaneously cleaved from the resin. The cleaved and deprotected peptide material is then precipitated, washed and dissolved in a buffer containing H2O/ACN/HOAC prior to lyophilization.
Peptide QC is determined using ESI mass spectrometry (confirming full length product) and reverse phase HPLC (RP-HPLC; determining the peptide purity. Peptides not meeting required purity specifications are purified by RP-HPLC.
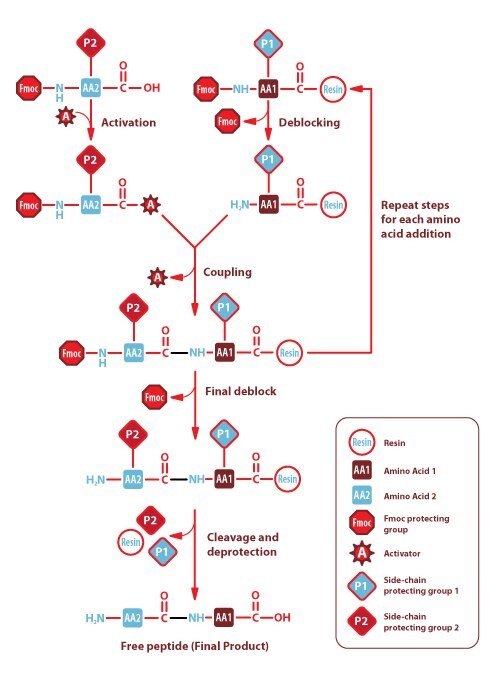
Figure 1.Solid Phase FMOC
Lot to Lot Variability
- Peptide purity - Peptide purity refers to the amount of desired peptide product relative to all other impurities, except moisture, as is determined by analytical HPLC. Peptide purity is the most common variability and may result in a high incidence of lot to lot variability. Peptides containing higher purity should exhibit less variability compared to their less pure counterparts. Peptides containing lower purity may contain differing amount of impurities for each synthesis lot. In some cases, peptide impurities with one or fewer amino acid deletions can still be active and contribute to the overall activity of the peptide.
- Aggregation - Hydrophobic peptides have a high tendency to aggregate. The activity of the peptide can greatly depend on which aggregate forms are still active. Peptides at high concentrations can also lead to higher aggregation.
- Chemical transformation - Peptides can undergo a series of chemical transformations, while in storage, that may lead to inactivity. Common examples include peptides containing Asp-Gly leading to iso-Asp formation, cross-linking of Cys containing peptides through the formation of disulfide bonds, and oxidation of Met to sulfoxides and pyroglutamate formation.
- Non-peptide impurities - Some impurities may be toxic to cells. If peptides are used for cell culture studies, Dithiotreitol (DTT) may be tolerated at less than 1 uM concentration in most cells. Lower purity peptides exposed to the common precipitation processes should have the majority of DTT removed from the final peptide material. RP-HPLC purified peptides are generally DTT-free.
Peptide Stability
Peptide stability is dependent on amino acid composition and sequence. Lyophilized peptide are generally more stable than those in solution. Storage of peptides in their lyophilized form at -20 oC or -80 oC will minimize degradation. Exposure to pH>8 and exposure to atmospheric oxygen should be minimized if possible. The following are potential degradation pathways for peptides:
- Hydrolysis: Generally a problem for peptides containing Asp (D). Avoid sequences containing the combination of Asp-Pro (D-P) or Asp-Gly (D-G) as these may undergo dehydration resulting in a cyclic imide intermediate. To a lesser extent, sequences containing Ser (S) may also form cyclic imide intermediates that can cleave the peptide chain.
- Oxidation: Cys (C) and Met (M) residues are the predominant amino acids that undergo reversible oxidation. Oxidation of cysteine is accelerated at higher pH, where the thiol is more easily deprotonated and readily forms intra-or inter-chain disulfide bonds. Disulfide bonds can be reversed by treatment with dethiothreitol (DTT) or tris(2-carboxyethylphosphine) hydrochloride (TCEP). Methionine residues oxidize by both chemical and photochemical pathways to form methionine sulfoxide and/or methionine sulfone, both of which are difficult to reverse.
- Diketopiperazine and pyroglutamic acid formation: Diketopiperazine formation typically occurs when Glycine (G) is in the third position from the N-terminus and more so if Pro (P) or Gly (G) is in position 1 or 2. The reaction involves a nucleophilic attack of the N-terminal nitrogen on the amide carbonyl between the second and third amino acid, resulting in the cleavage of the first 2 amino acids in the form of a diketopiperazine. Pyroglutamic acid formation is almost inevitable if Gln (Q) is located at the N-terminal position of the sequence. The N-terminal nitrogen attacks the side chain carbonyl carbon of the Gln (Q) to form a deaminated pyroglutamayl peptide analog. The conversion may also occur in peptide sequences containing Asn (N) at the N-terminal position, but to a much lesser extent.
To continue reading please sign in or create an account.
Don't Have An Account?