LC/MS (TOF) Analysis of Antiarrhythmic Drugs and Metabolites in Plasma on Ascentis® Express HILIC after Sample Prep using HybridSPE®-Phospholipid
Materials
Analytical column
SPE tube or plate
Standard
CONDITIONS
sample preparation
SPE (Solid Phase Extraction)
sample/matrix
rat plasma stabilized with K2EDTA was spiked directly from stock standard to a level of 400 ng/mL
SPE tube/cartridge
HybridSPE-Phospholipid 96-Well Plate, 50 mg/2 mL (575656-U)
sample addition
apply 100 μL of plasma to plate, followed by 300 μL of 1% formic acid acetonitrile. Agitate via vortex for 4 min
elution
place on vacuum manifold and apply 10” Hg vacuum for 4 minutes. Collect filtrate and analyze directly.
column
Ascentis Express HILIC, 10 cm x 2.1 mm I.D., 2.7 μm particles (53939-U)
mobile phase
[A] 5 mM ammonium formate; [B] 5 mM ammonium formate in acetonitrile; (5:95, A:B, pH 7.0 with formic acid)
flow rate
0.4 mL/min
pressure
1305 psi (90 bar)
column temp.
35 °C
detector
ESI(+), full scan, m/z 100-1000
injection
0.5 μL
sample
plasma extract, analyte concentration of final sample work up is equivalent to 100 ng/mL
Description
Analysis Note
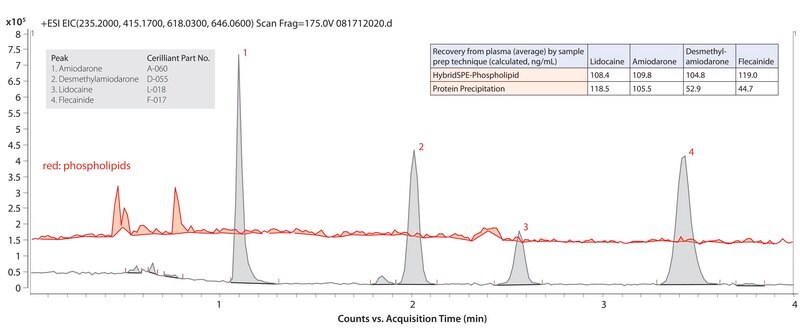
The basic characteristics of these compounds make them targets for HILIC chromatographic separation. HILIC mobile phases consist of a high composition of acetonitrile, which facilitates the direct analysis of precipitated plasma samples without the need for additional sample solvent exchange. In most cases, the high organic mobile phase also facilitates increased analyte response in ESI(+) MS detections. This application used HybridSPE-Phospholipid sample prep to remove phospholipids and precipitated proteins prior to LC/MS analysis on an Ascentis Express HILIC Fused-Core HPLC column. The highest grade solvents provided clean, robust operation. Cerilliant CRMs provided reliable quantification. The resulting method was robust and sensitive.
Legal Information
Ascentis is a registered trademark of Merck KGaA, Darmstadt, Germany
HybridSPE is a registered trademark of Merck KGaA, Darmstadt, Germany