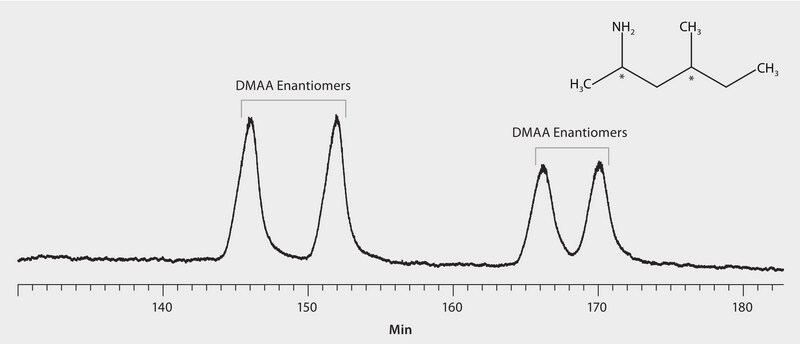
GC Analysis of 1,3-Dimethylamylamine (DMAA) Enantiomers (N-Pentafluoropropionyl Derivatives) on Astec® CHIRALDEX™ G-DM, 30 ºC Oven Temp.
Materials
Analytical column
Standard
mobile phase component
CONDITIONS
column
Astec CHIRALDEX G-DM, 30 m x 0.25 mm I.D., 0.12 μm particles (77033AST)
oven
30 °C (isothermal)
inj. temp.
250 °C
detector
FID, 250 °C
carrier gas
helium, 1 mL/min, constant
injection
1.0 μL 100:1 split
Description
Analysis Note
1,3-Dimethylamylamine (DMAA) is a stimulant found in various health supplements. Although often labelled as "natural," derived from geranium plants, it is suspected of actually having synthetic origins. If synthetic, then the supplement would fall under FDA regulations. In this study, chiral GC was used to measure the enantiomeric and diastereomeric ratios of synthetic DMAA. Shown here are the two sets of enantiomers of DMAA separated by GC on Astec CHIRALDEX G-DM.
Other Notes
10 mg of DMAA standard and 10 mg of internal standard (2-aminopentane) were dissolved in 0.5 mL of dichloromethane. 0.5 mL PFPA was added to the vial and the vial was sealed with a silicone rubber insert. The solution was heated for 30 min at 50 °C. Then the solvent and residual PFPA were removed at room temperature under reduced pressure. The derivatized DMAA and internal standard were transferred to a 10 mL volumetric flask and diluted to 10 mL with heptane.
Legal Information
Astec is a registered trademark of Merck KGaA, Darmstadt, Germany
CHIRALDEX is a trademark of Sigma-Aldrich Co. LLC