1238501
USP
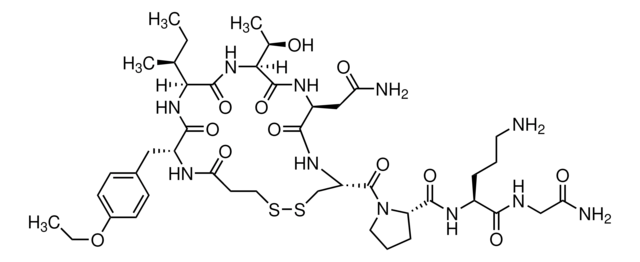
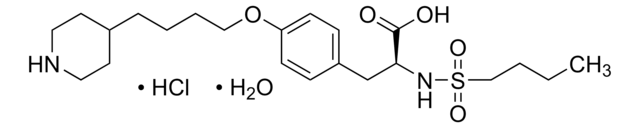
Eptifibatide
United States Pharmacopeia (USP) Reference Standard
Synonym(s):
N6-Amidino-N2-(3-mercaptopropionyl)-L-lysylglycyl-L-α-aspartyl-L-tryptophyl-L-prolyl-L-cysteinamide, cyclic(1-6)-disulfide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
41116107
NACRES:
NA.24
Recommended Products
grade
pharmaceutical primary standard
agency
certified by the USP
API family
eptifibatide
manufacturer/tradename
USP
application(s)
pharmaceutical
format
neat
storage temp.
−20°C
General description
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
Application
Eptifibatide USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia.
Analysis Note
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
Other Notes
Sales restrictions may apply
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service