565372-U
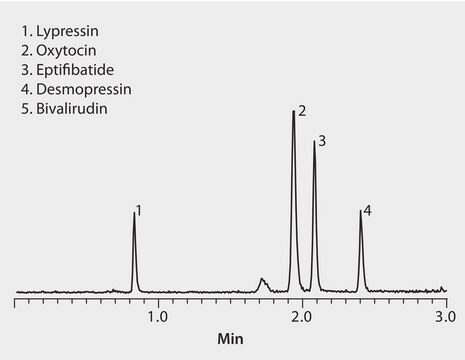
Ascentis® RP-Amide (5 µm) HPLC Columns
L × I.D. 2 cm × 2.1 mm Supelguard Guard Cartridge pkg of 2 ea, Guard Cartridge holder required for use
About This Item
Recommended Products
product name
Ascentis® RP-Amide Supelguard Guard Cartridge, 5 μm particle size, L × I.D. 2 cm × 2.1 mm, pkg of 2 ea
material
stainless steel column
agency
suitable for USP L60
product line
Ascentis®
feature
endcapped
packaging
pkg of 2 ea
extent of labeling
19.5% Carbon loading
parameter
<70 °C temp. range
technique(s)
HPLC: suitable
LC/MS: suitable
L × I.D.
2 cm × 2.1 mm
surface area
450 m2/g
impurities
<5 ppm metals
matrix
fully porous particle
matrix active group
amide, alkyl phase
particle size
5 μm
pore size
100 Å
operating pH range
2-8
application(s)
food and beverages
separation technique
reversed phase
Looking for similar products? Visit Product Comparison Guide
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service