48320-U
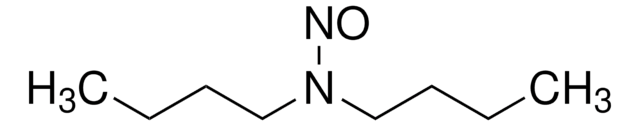
N-Nitrosodi-n-butylamine solution
certified reference material, 2000 μg/mL in methylene chloride
Synonym(s):
Dibutyl nitrosamine
About This Item
Recommended Products
grade
certified reference material
Quality Level
CofA
current certificate can be downloaded
packaging
ampule of 1 mL
concentration
2000 μg/mL in methylene chloride
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
environmental
format
single component solution
storage temp.
2-30°C
SMILES string
N(N=O)(CCCC)CCCC
InChI
1S/C8H18N2O/c1-3-5-7-10(9-11)8-6-4-2/h3-8H2,1-2H3
InChI key
YGJHZCLPZAZIHH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
signalword
Warning
hcodes
Hazard Classifications
Carc. 2 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Central nervous system
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Certificates of Analysis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Learn about LC-MS/MS method development to quantify NDMA impurity in valsartan drug substance using Titan™ C18 column based UHPLC separation
Nitrosamines have been discovered as a serious contaminant group in active pharmaceutical ingredients (API) belonging to the sartan family. This article describes a GC-MS method for the determination of nitrosamines in Valsartan tablets according to US FDA guide lines that can be used for pharma QC.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service