PZ0243
PF-06672131
≥95% (HPLC)
Synonym(s):
(2E)-N-[4-[(3-Chloro-4-fluorophenyl)amino]-7-(2-propyn-1-yloxy)-6-quinazolinyl]-4-(dimethylamino)-2-butenamide, (E)-N-(4-((3-Chloro-4-fluorophenyl)amino)-7-(prop-2-yn-1-yloxy)quinazolin-6-yl)-4-(dimethylamino)but-2-enamide, Probe 8
About This Item
Recommended Products
Quality Level
assay
≥95% (HPLC)
form
powder
color
white to beige
solubility
DMSO: 5 mg/mL, clear (warmed)
storage temp.
room temp
SMILES string
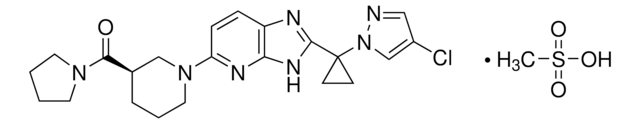
FC(C=C1)=C(Cl)C=C1NC2=NC=NC3=CC(OCC#C)=C(NC(/C=C/CN(C)C)=O)C=C32
Biochem/physiol Actions
signalword
Warning
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Related Content
The aim of the Cravatt research group is to understand the roles that mammalian enzymes play in physiological and pathological processes and to use this knowledge to identify novel therapeutic targets for the treatment of human disease. To achieve these goals, they develop and apply new technologies that bridge the fields of chemistry and biology, ascribing to the philosophy that the most significant biomedical problems require creative multidisciplinary approaches for their solution. The group's technological innovations address fundamental challenges in systems biology that are beyond the scope of contemporary methods. For instance, enzymes are tightly regulated by post-translational events in vivo, meaning that their activity may not correlate with expression as measured by standard genomic and proteomic approaches. Considering that it is an enzyme's activity, rather than abundance that ultimately dictates its role in cell physiology and pathology, the Cravatt group has introduced a set of proteomic technologies that directly measures this parameter. These activity-based protein profiling (ABPP) methods exploit the power of chemistry to engender new tools and assays for the global analysis of enzyme activities. The enzyme activity profiles generated by ABPP constitute unique molecular portraits of cells and tissues that illuminate how metabolic and signaling networks are regulated in vivo. Additionally, by evaluating enzymes based on functional properties rather than mere abundance, ABPP acquires high-content proteomic information that is enriched in novel markers and targets for the diagnosis and treatment of human disease.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service