MSP12
Membrane Scaffold Protein 2N2
recombinant, expressed in E. coli, MSP1D1-MSP1D2 fusion protein
Synonym(s):
Membrane scaffold protein
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
UNSPSC Code:
12352200
NACRES:
NA.26
Recommended Products
recombinant
expressed in E. coli
Quality Level
assay
≥90% (SDS-GE)
form
buffered aqueous solution
mol wt
45,541.2 Da
solubility
water: soluble
shipped in
ambient
storage temp.
−20°C
General description
Research area: Cell Struc
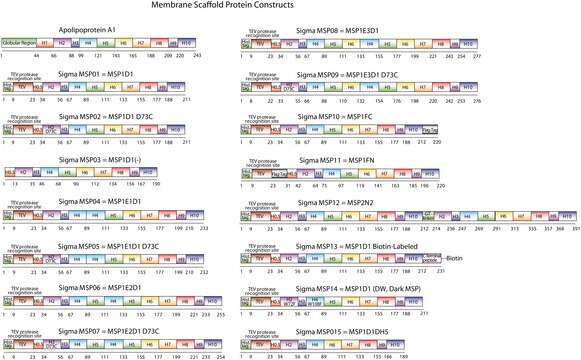
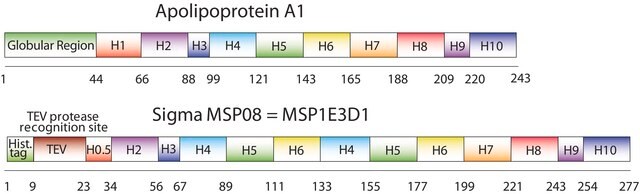
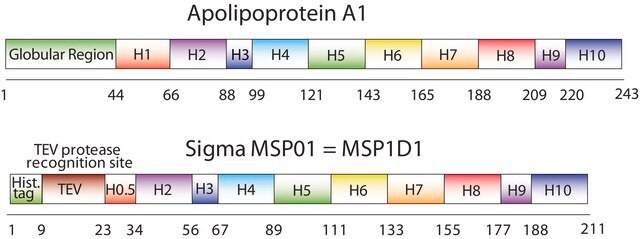
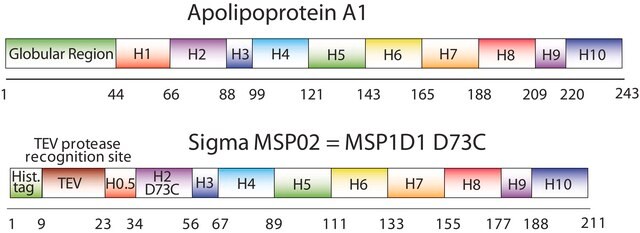
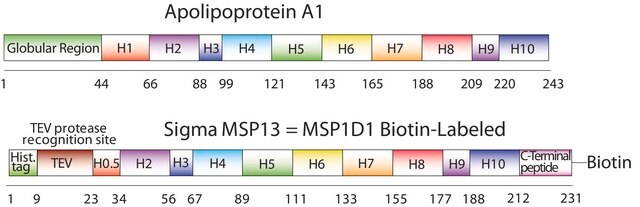
The first MSP, MSP1, was engineered with its sequence based on the sequence of A-1 but without the globular N-terminal domain of native A-1. The Membrane Scaffold Protein 1D1 (MSP1D1) variant of MSP1 deletes the first 11 amino acids in the Helix 1 portion (referred to as “H0.5” in the accompanying figure) of the original MSP1 sequence. Membrane Scaffold Protein 2N2 (MSP 2N2) is a fusion of MSP1D1 and another MSP variant, MSP1D2. MSP1D2 deletes the first 22 amino acids of the original MSP sequence (i.e. the entire H1 segment). In MSP2N2, a GT linker connects MSP1D1 and MSP1D2.
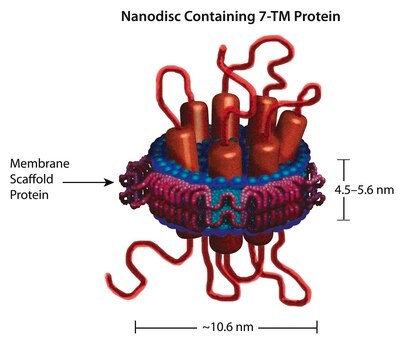
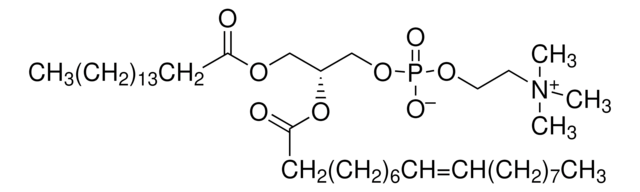
Nanodisc technology is an approach rendering membrane proteins soluble in aqueous solutions in a native-like bilayer environment, where the membrane proteins remain stable and active. The Nanodisc concept is derived from high-density lipoprotein (HDL) particles and their primary protein component, apolipoprotein. The Nanodisc is a non-covalent structure of phospholipid bilayer and membrane scaffold protein (MSP), a genetically engineered protein that mimics the function of Apolipoprotein A-1 (ApoA-1).
The first MSP, MSP1, was engineered with its sequence based on the sequence of A-1 but without the globular N-terminal domain of native A-1. The Membrane Scaffold Protein 1D1 (MSP1D1) variant of MSP1 deletes the first 11 amino acids in the Helix 1 portion (referred to as “H0.5” in the accompanying figure) of the original MSP1 sequence. Membrane Scaffold Protein 2N2 (MSP 2N2) is a fusion of MSP1D1 and another MSP variant, MSP1D2. MSP1D2 deletes the first 22 amino acids of the original MSP sequence (i.e. the entire H1 segment). In MSP2N2, a GT linker connects MSP1D1 and MSP1D2.
Nanodisc technology is an approach rendering membrane proteins soluble in aqueous solutions in a native-like bilayer environment, where the membrane proteins remain stable and active. The Nanodisc concept is derived from high-density lipoprotein (HDL) particles and their primary protein component, apolipoprotein. The Nanodisc is a non-covalent structure of phospholipid bilayer and membrane scaffold protein (MSP), a genetically engineered protein that mimics the function of Apolipoprotein A-1 (ApoA-1).
Application
Membrane Scaffold Protein 2N2 has been used in enzyme-linked immunosorbent assay (ELISA) and for reconstitution of human β3 homopentameric gamma-aminobutyric acid type A receptor (GABAAR) in nanodiscs.
Biochem/physiol Actions
Scaffold proteins play a crucial role in providing specificity to the mitogen-activated protein kinase (MAPK) pathway, which is essential for normal cellular functions and development. They help regulate the localization of MAPK components, allowing for targeted modulation of cellular responses without affecting global MAPK activity.
Legal Information
Nanodisc technology, and many of its uses, are covered by the following patents held by the University of Illinois.
- 7,691,414 Membrane scaffold proteins
- 7,662,410 Membrane scaffold proteins and embedded membrane proteins
- 7,622,437 Tissue factor compositions and methods
- 7,592,008 Membrane scaffold proteins
- 7,575,763 Membrane scaffold proteins and tethered membrane proteins
- 7,083,958 Membrane scaffold proteins
- 7,048,949 Membrane scaffold proteins
Storage Class
12 - Non Combustible Liquids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tomasz Uchański et al.
Nature methods, 18(1), 60-68 (2021-01-08)
Nanobodies are popular and versatile tools for structural biology. They have a compact single immunoglobulin domain organization, bind target proteins with high affinities while reducing their conformational heterogeneity and stabilize multi-protein complexes. Here we demonstrate that engineered nanobodies can also
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service