L8906
Lipase from Mucor javanicus
lyophilized powder, ≥300 units/mg solid (using olive oil)
Synonym(s):
Triacylglycerol acylhydrolase, Triacylglycerol lipase
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
form
lyophilized powder
Quality Level
specific activity
≥300 units/mg solid (using olive oil)
storage temp.
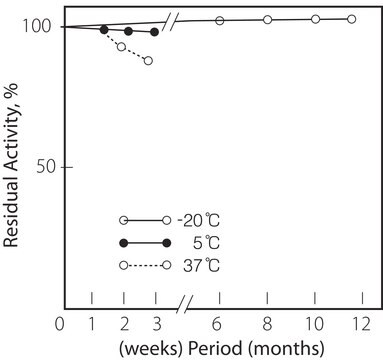
2-8°C
InChI
1S/C11H9N3O2.Na/c15-8-4-5-9(10(16)7-8)13-14-11-3-1-2-6-12-11;/h1-7,16H,(H,12,14);/q;+1/b13-9-;
InChI key
QWZUIMCIEOCSJF-CHHCPSLASA-N
Looking for similar products? Visit Product Comparison Guide
General description
Lipases are hydrolytic enzymes which break down triacylglycerides into free fatty acids and glycerols.
Application
Lipase from Mucor javanicus can be used to catalyze the production of polyglycerol polyricinoleate from polyricinoleic acid and polyglycerol-3.
Lipase from Mucor javanicus has been used in a study to assess the effects of lyophilization time and water content on the salt-induced activation of enzymes in organic solvents.
Biochem/physiol Actions
Tri-, di-, and monoglycerides are hydrolyzed (in decreasing order of rate).
Unit Definition
One unit will hydrolyze 1.0 microequivalent of fatty acid from a triglyceride in 1 hr at pH 7.7 at 37 °C using olive oil (30 minute incubation).
Analysis Note
Protein determined by biuret.
signalword
Danger
hcodes
pcodes
Hazard Classifications
Resp. Sens. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Screening and selection of lipases for the enzymatic production of polyglycerol polyricinoleate.
Bo?dalo, A.
Biochemical Engineering Journal, 46, 217-222 (2009)
M T Ru et al.
Biotechnology and bioengineering, 63(2), 233-241 (1999-04-01)
The addition of simple inorganic salts to aqueous enzyme solutions prior to lyophilization results in a dramatic activation of the dried powder in organic media relative to enzyme with no added salt. Activation of both the serine protease subtilisin Carlsberg
Hani El-Nezami et al.
Journal of agricultural and food chemistry, 52(14), 4577-4581 (2004-07-09)
Viable, heat-and acid-killed Lactobacillus rhamnosus strain GG (LGG) has shown high binding properties with zearalenone (ZEN). To identify the type of chemical moieties and interactions involved in binding with the ZEN, LGG was subjected to different chemical and enzymatical treatments
A M Stepan et al.
Journal of biotechnology, 167(1), 16-23 (2013-06-19)
This is the first report on successful enzyme catalyzed surface esterification of hemicellulose films. Enzyme catalyzed surface acetylation with vinyl acetate and stearation with vinyl stearate were studied on rye arabinoxylan (AX) films. Different surface analytical techniques (FT-IR, TOF-SIMS, ESCA
Hidehiko Wakabayashi et al.
Journal of agricultural and food chemistry, 51(15), 4349-4355 (2003-07-10)
The enantioselectivity of the generation of 3-mercaptohexanal and 3-mercaptohexanol, two potent sulfur-containing aroma compounds, by lipase-catalyzed hydrolysis of the corresponding 3-acetylthioesters was investigated. The stereochemical course of the kinetic resolutions was followed by capillary gas chromatography using modified cyclodextrins as
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service