L7022
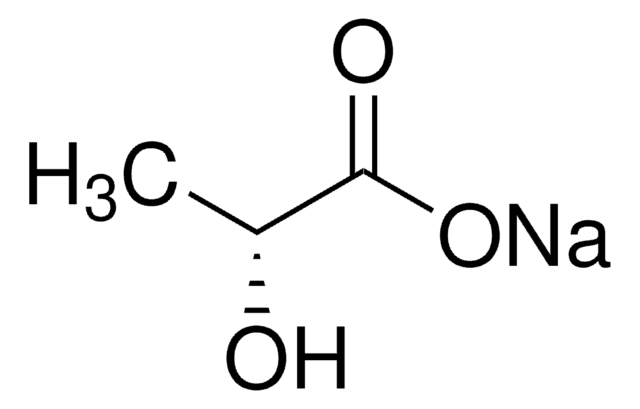
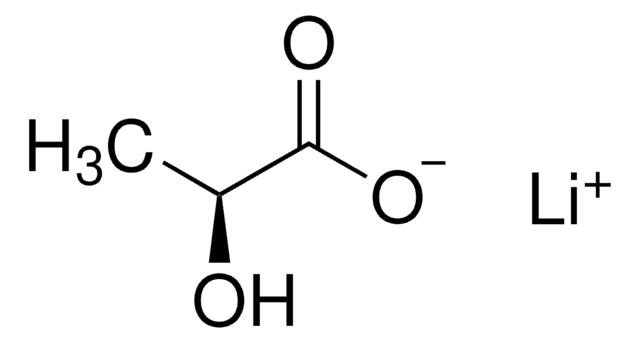
Sodium L-lactate
~98%
Synonym(s):
(S)-2-Hydroxypropionic acid sodium salt, L-Lactic acid sodium salt, Sarcolactic acid sodium salt
About This Item
Recommended Products
biological source
synthetic (chemical)
Quality Level
assay
~98%
form
powder or crystals
color
white to faint yellow
mp
163-165 °C (lit.)
solubility
water: 50 mg/mL, clear, colorless
storage temp.
2-8°C
SMILES string
[Na+].C[C@H](O)C([O-])=O
InChI
1S/C3H6O3.Na/c1-2(4)3(5)6;/h2,4H,1H3,(H,5,6);/q;+1/p-1/t2-;/m0./s1
InChI key
NGSFWBMYFKHRBD-DKWTVANSSA-M
Looking for similar products? Visit Product Comparison Guide
General description
This multifaceted nature of Sodium L-lactate positions it as a valuable molecule for research in cellular metabolism, neuroprotection, antimicrobial, and nutritional science, where its diverse properties, including efficient energy conversion, neuroprotective effects, and potent antimicrobial activity contribute to a deeper understanding across these scientific domains.
Application
- as a medium supplement and cell fuel source for human mammary epithelial cell line(MCF10A) and dendritic cell culture
- as a gluconeogenic substrate in hepatic glucose production assay in primary hepatocytes
- in the glucose production medium for glucose production assay in human embryonic kidney (HEK293T) cells
- as a standard for calibration in lactate assay in bone marrow-derived macrophages
Biochem/physiol Actions
Features and Benefits
Other Notes
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Sigma-Aldrich presents an article about how proliferatively active cells require both a source of carbon and of nitrogen for the synthesis of macromolecules. Although a large proportion of tumor cells utilize aerobic glycolysis and shunt metabolites away from mitochondrial oxidative phosphorylation, many tumor cells exhibit increased mitochondrial activity.
We presents an article about the Warburg effect, and how it is the enhanced conversion of glucose to lactate observed in tumor cells, even in the presence of normal levels of oxygen. Otto Heinrich Warburg demonstrated in 1924 that cancer cells show an increased dependence on glycolysis to meet their energy needs, regardless of whether they were well-oxygenated or not.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service