G7141
Glucose Oxidase from Aspergillus niger
Type X-S, lyophilized powder, 100,000-250,000 units/g solid (without added oxygen)
Synonym(s):
β-D-Glucose:oxygen 1-oxidoreductase, G.Od., GOx
About This Item
Recommended Products
type
Type X-S
Quality Level
form
lyophilized powder
specific activity
100,000-250,000 units/g solid (without added oxygen)
mol wt
160 kDa
composition
Protein, ≥65%
greener alternative product characteristics
Waste Prevention
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
application(s)
diagnostic assay manufacturing
foreign activity
Catalase ≤5 units/mg protein
greener alternative category
storage temp.
−20°C
InChI
1S/C6H12O6/c7-1-2-3(8)4(9)5(10)6(11)12-2/h2-11H,1H2/t2-,3-,4+,5-,6-/m1/s1
InChI key
WQZGKKKJIJFFOK-VFUOTHLCSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
pI: 4.2
Extinction coefficient: E1% = 16.7 (280 nm)
Glucose oxidase from Aspergillus niger is a dimer consisting of 2 equal subunits with a molecular mass of 80 kDa each. Each subunit contains one flavin adenine dinulceotide moiety and one iron. The enzyme is a glycoprotein containing ~16% neutral sugar and 2% amino sugars. The enzyme also contains 3 cysteine residues and 8 potential sites for N-linked glycosylation.
Glucose oxidase is capable of oxidizing D-aldohexoses, monodeoxy-D-glucoses, and methyl-D-glucoses at varying rates.
The pH optimum for glucose oxidase is 5.5, while it has a broad activity range of pH 4-7. Glucose oxidase is specific for β-D-glucose with a KM of 33-110 mM.
Glucose oxidase does not require any activators, but it is inhibited by Ag+, Hg2+, Cu2+, phenylmercuric acetate, and p-chloromercuribenzoate. It is not inhibited by the nonmetallic SH reagents: N-ethylmaleimide, iodoacetate, and iodoacetamide.
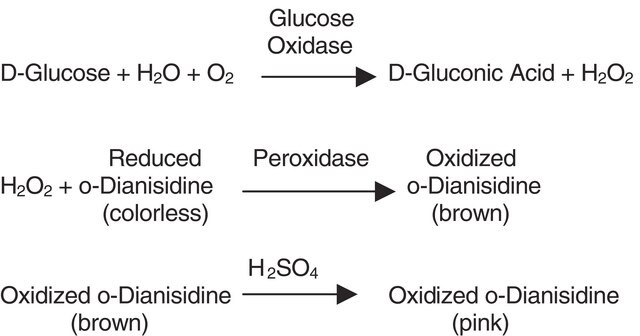
Glucose oxidase can be utilized in the enzymatic determination of D-glucose in solution. As glucose oxidase oxidizes β-D-glucose to D-gluconolactate and hydrogen peroxide, horseradish peroxidase is often used as the coupling enzyme for glucose determination. Although glucose oxidase is specific for β-D-glucose, solutions of D-glucose can be quantified as α-D-glucose will mutorotate to β-D-glucose as the β-D-glucose is consumed by the enzymatic reaction.
Application
- in the (glucose oxidase) GO reagent to measure the glucose content by the glucose oxidase (GO) method
- to activate the human renal carcinoma cell line for constructing the oxidative stress model
- to study its influence in the paste on the analytical performance of the bioelectrode
Biochem/physiol Actions
Quality
Unit Definition
Analysis Note
signalword
Danger
hcodes
pcodes
Hazard Classifications
Resp. Sens. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service