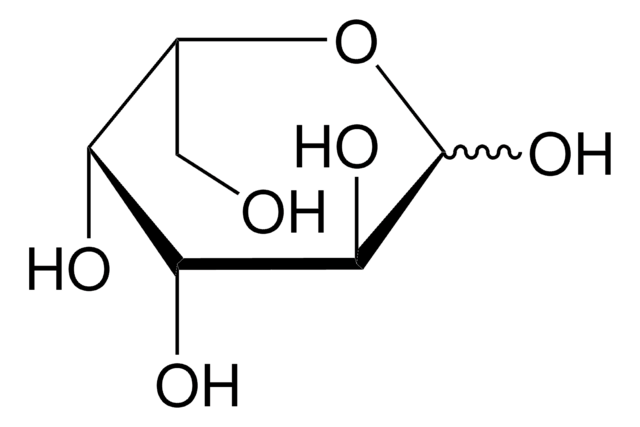
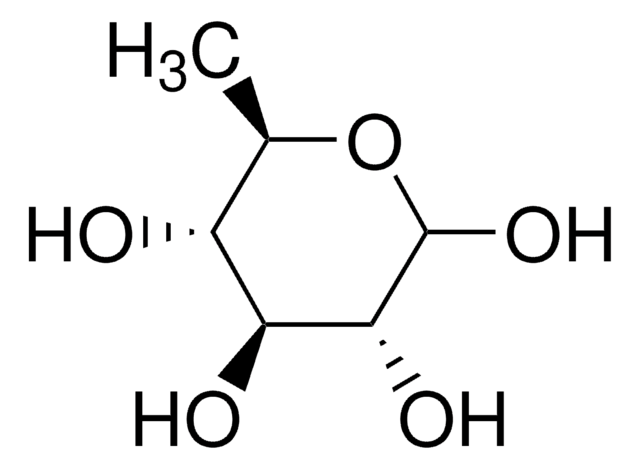
F2252
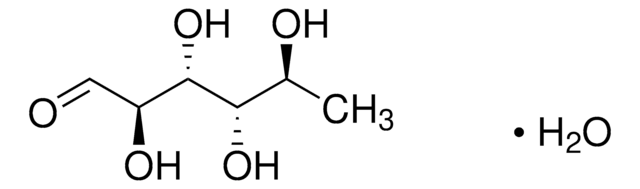
L-(−)-Fucose
≥98% (GC)
Synonym(s):
6-Deoxy-L-galactose
Sign Into View Organizational & Contract Pricing
All Photos(5)
About This Item
Empirical Formula (Hill Notation):
C6H12O5
CAS Number:
Molecular Weight:
164.16
Beilstein/REAXYS Number:
1723321
EC Number:
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
Quality Level
assay
≥98% (GC)
form
powder
technique(s)
gas chromatography (GC): suitable
color
white
mp
150-153 °C (lit.)
solubility
H2O: soluble 50 mg/mL, clear to very slightly hazy
SMILES string
C[C@H](O)[C@@H](O)[C@@H](O)[C@H](O)C=O
InChI
1S/C6H12O5/c1-3(8)5(10)6(11)4(9)2-7/h2-6,8-11H,1H3/t3-,4+,5+,6-/m0/s1
InChI key
PNNNRSAQSRJVSB-KCDKBNATSA-N
Looking for similar products? Visit Product Comparison Guide
General description
L-Fucose, a natural monosaccharide found in mammals, is an important component of several N- and O-linked glycans and glycolipids.
Application
L-(−)-Fucose has been used:
- as the non-haptenic sugar in lectin bead-binding assay to study its effects on the binding of immobilized lectins like peanut agglutinin (PNA) and Dolichos biflorus agglutinin (DBA)
- as an internal standard to dilute the enzymatic hydrolysates for analytical methods
- in the synthesis of fucosyl thioglycoside
Biochem/physiol Actions
L-Fucose (6-Deoxy-L-galactose) is used in studies of fucoidan polysaccharides containing glycans. It is studied as a glycan modifying carbohydrate that generates antigenic sites recognized by IgE antibodies. L-Fucose is used as a substrate to identify, differentiate, and characterize enzymes such as fucosidase(s),l-fucose isomerase(s), and L-fucose dehydrogenase(s). It may be used to study organelles, and bacterial microcompartments, involved in the degradation of plant and algal cell wall sugars. L-Fucose may also be used as a reference compound in rare sugar identification and analysis.
L-Fucose is broadly used as a food additive. It possesses anti-inflammatory effects. It can inhibit the cutaneous immune reaction and alveolar macrophage priming. L-fucose can also suppress tumor growth and tumorigenesis.
Other Notes
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Marcel Tutor Ale et al.
Marine drugs, 9(10), 2106-2130 (2011-11-11)
Seaweeds--or marine macroalgae--notably brown seaweeds in the class Phaeophyceae, contain fucoidan. Fucoidan designates a group of certain fucose-containing sulfated polysaccharides (FCSPs) that have a backbone built of (1→3)-linked α-L-fucopyranosyl or of alternating (1→3)- and (1→4)-linked α-L-fucopyranosyl residues, but also include
Jay J Listinsky et al.
American journal of translational research, 3(4), 292-322 (2011-09-10)
Breast cancer cells incorporate the simple sugar alpha-L-fucose (fucose) into glycoproteins and glycolipids which, in turn, are expressed as part of the malignant phenotype. We have noted that fucose is not simply a bystander molecule, but, in fact, contributes to
Krzysztof Brzezinski et al.
Acta crystallographica. Section D, Biological crystallography, 68(Pt 2), 160-168 (2012-01-28)
Rhizobial NodZ α1,6-fucosyltransferase (α1,6-FucT) catalyzes the transfer of the fucose (Fuc) moiety from guanosine 5'-diphosphate-β-L-fucose to the reducing end of the chitin oligosaccharide core during Nod-factor (NF) biosynthesis. NF is a key signalling molecule required for successful symbiosis with a
Simon N Robinson et al.
Experimental hematology, 40(6), 445-456 (2012-02-07)
Delayed engraftment remains a major hurdle after cord blood (CB) transplantation. It may be due, at least in part, to low fucosylation of cell surface molecules important for homing to the bone marrow microenvironment. Because fucosylation of specific cell surface
Karineh Petrossian et al.
Acta histochemica, 109(6), 491-500 (2007-08-21)
By using a non-cancer and a cancer cell line originally from the same tissue (colon), coupled with testing lectins for cell binding and for their effects on these cell lines in culture, this study describes a simple multi-parameter approach that
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service