E6375
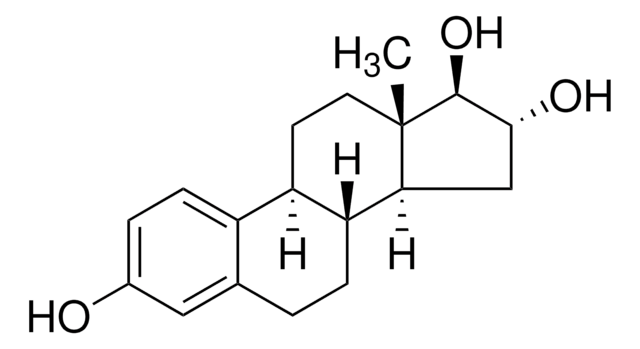
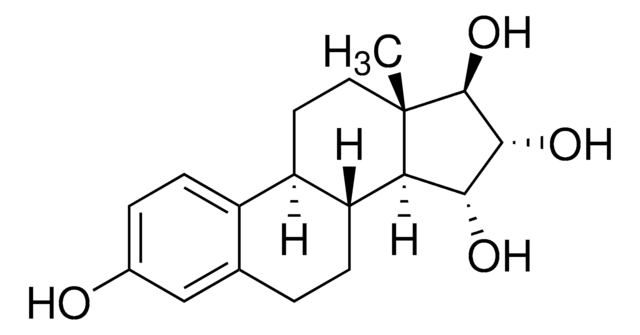
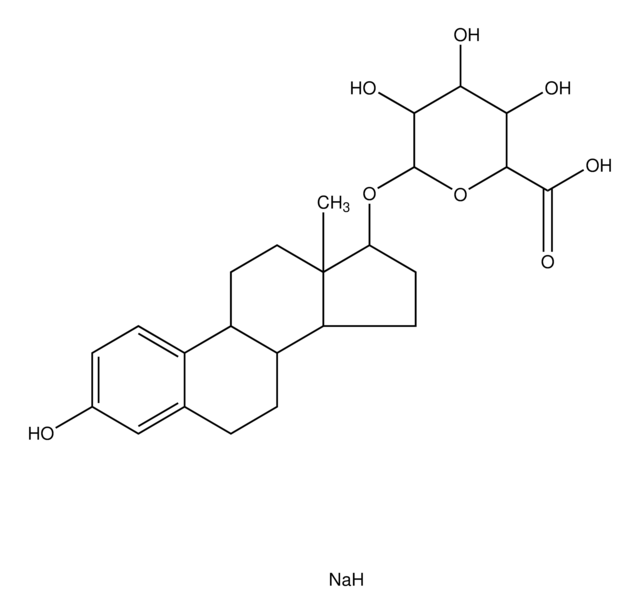
Estriol 3-sulfate sodium salt
≥98% (TLC)
Synonym(s):
1,3,5(10)-Estratriene-3,16α,17β-triol 3-sulfate, 3,16α,17β-Trihydroxy-1,3,5(10)-estratriene 3-sulfate
About This Item
Recommended Products
Quality Level
assay
≥98% (TLC)
form
powder
contains
~30% methylglucamine as stabilizer
solubility
methanol: 19.60-20.40 mg/mL, clear, colorless to faintly yellow
application(s)
diagnostic assay manufacturing
hematology
histology
shipped in
ambient
storage temp.
room temp
SMILES string
[Na].[H][C@]12CC[C@]3(C)[C@@H](O)[C@H](O)C[C@@]3([H])[C@]1([H])CCc4cc(OS(O)(=O)=O)ccc24
InChI
1S/C18H24O6S.Na.H/c1-18-7-6-13-12-5-3-11(24-25(21,22)23)8-10(12)2-4-14(13)15(18)9-16(19)17(18)20;;/h3,5,8,13-17,19-20H,2,4,6-7,9H2,1H3,(H,21,22,23);;/t13-,14-,15+,16-,17+,18+;;/m1../s1
InChI key
NGMCHEUPKPRHNC-XZEIGEQDSA-N
Packaging
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Separation of Estriol 3-(β-D-glucuronide) sodium salt; β-Estradiol 3-(β-D-glucuronide) 17-sulfate dipotassium salt; Estriol 3-sulfate sodium salt; β-Estradiol 3,17-disulfate dipotassium salt, ≥95%; β-Estradiol 17-(β-D-glucuronide) sodium salt; β-Estradiol 3-(β-D-glucuronide) sodium salt; Estrone 3-(β-D-glucuronide) sodium salt; β-Estradiol 3-sulfate sodium salt, ≥93%; Estriol, ≥97%; Estrone 3-sulfate sodium salt, contains ~35% Tris as stabilizer; β-Estradiol, ≥98%; α-Estradiol, powder, ≥98% (TLC); Estrone, ≥99%
The Titan C18 column provided efficient and rapid resolution of thirteen related estrogenic compounds. Ultra Ultra high purity solvents provided robust operation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service