C9756
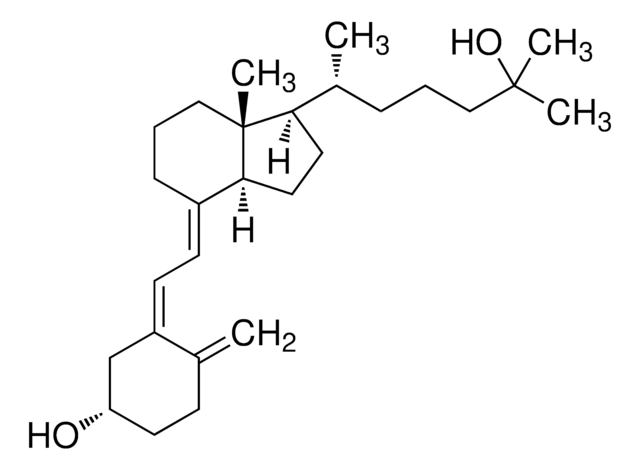
Cholecalciferol
≥98% (HPLC)
Synonym(s):
(+)-Vitamin D3, 7-Dehydrocholesterol activated, Activated 7-dehydrocholesterol, Calciol
About This Item
Recommended Products
biological source
synthetic (organic)
Quality Level
assay
≥98% (HPLC)
form
powder
technique(s)
HPLC: suitable
color
white to off-white
mp
83-86 °C (lit.)
storage temp.
2-8°C
SMILES string
CC(C)CCC[C@@H](C)[C@@]1([H])CC[C@@]([C@]1(C)CCC/2)([H])C2=C\C=C(C[C@@H](O)CC3)/C3=C
InChI
1S/C27H44O/c1-19(2)8-6-9-21(4)25-15-16-26-22(10-7-17-27(25,26)5)12-13-23-18-24(28)14-11-20(23)3/h12-13,19,21,24-26,28H,3,6-11,14-18H2,1-2,4-5H3/b22-12+,23-13-/t21-,24+,25-,26+,27-/m1/s1
InChI key
QYSXJUFSXHHAJI-YRZJJWOYSA-N
Gene Information
human ... VDR(7421)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Alterations in regulators of the renal-bone axis, inflammation and iron status in older people with early renal impairment and the effect of vitamin D supplementation.: This study delves into the significant roles of cholecalciferol in modulating the renal-bone axis, inflammatory responses, and iron metabolism in elderly patients with renal impairment. Through vitamin D supplementation, researchers observed pivotal changes that could guide future therapeutic strategies (Christodoulou M et al., 2024).
- Investigating the effects of 25-hydroxyvitamin D3 on clinical outcomes in multiple sclerosis patients: A randomized, double-blind clinical trial- a pilot study.: This clinical trial assesses the efficacy of cholecalciferol in improving the health outcomes of patients with multiple sclerosis. The use of cholecalciferol potentially enhances neurological function and reduces disease progression, illustrating its vital role in neurodegenerative disease management (Maghbooli Z et al., 2024).
- Vitamin D regulates COVID-19 associated severity by suppressing the NLRP3 inflammasome pathway.: This pivotal research links cholecalciferol to the reduction of COVID-19 severity through its regulatory effects on the NLRP3 inflammasome pathway. The findings underscore the potential of vitamin D as a modulatory agent in the immune response against viral infections (Khalil B et al., 2024).
- Effects of Vitamin D Supplementation on Central Hemodynamic Parameters and Autonomic Nervous System in Obese or Overweight Individuals.: This randomized controlled trial explores how cholecalciferol supplementation can affect cardiovascular and autonomic functions in obese or overweight patients, providing insights into its benefits beyond bone health and into cardiovascular regulation (Faria ACC et al., 2024).
Biochem/physiol Actions
Storage and Stability
Related product
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 2 Dermal - Acute Tox. 2 Inhalation - Acute Tox. 2 Oral - STOT RE 1 Oral
Storage Class
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Vitamin D2 (ergocalciferol) is naturally synthesized from ergosterol by invertebrates, fungi, and plants in response to ultraviolet B irradiation, while vitamin D3 synthesis (cholecalciferol) is uniquely initiated in the skin of vertebrates. During sun exposure, ultraviolet B photons are absorbed by 7-dehydrocholesterol, which is found within the plasma membranes of epidermal and dermal skin layers. This reaction yields an unstable derivative of 7-dehydrocholesterol, named precholecalcitrol, which rapidly rearranges to vitamin D3. Vitamin D binding protein (DBP) is a carrier protein responsible for drawing vitamin D3 from the plasma membrane into the dermal capillaries within the extracellular space.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service