C6607
Caspase 10 human
>90% (SDS-PAGE), recombinant, expressed in E. coli, lyophilized powder, 8,000 units/mg protein (Bradford)
Synonym(s):
Flice2, Mch4
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
recombinant
expressed in E. coli
Quality Level
assay
>90% (SDS-PAGE)
form
lyophilized powder
specific activity
8,000 units/mg protein (Bradford)
mol wt
~30 kDa
solubility
PBS: soluble
UniProt accession no.
shipped in
dry ice
storage temp.
−70°C
Gene Information
human ... CASP10(843)
Biochem/physiol Actions
Caspase 10 has two death effector domains (DEDs) that bind to the DED in the adapter molecule FADD and recruits both TNFR1 and CD95 to form complexes with these receptors. Caspase 10 cleaves and activates caspases 3, 4, 6, 7, 8 and 9 which causes the proteolytic cleavage of many key proteins such as PARP. Based on gene expression studies, caspase 10 may be crucial in embryonic development. In view of its structural homology to caspase 8, the initiator caspase in death receptor-mediated apoptosis, caspase 10 may function in a similar and redundant manner.
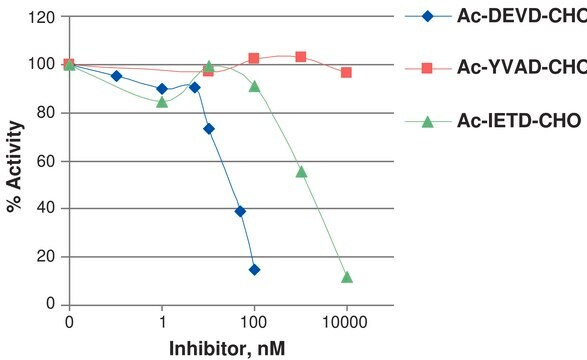
Unit Definition
One unit will hydrolyze 1 nmol of the caspase substrate IETD-pNA to IETD and p-nitroaniline per hour at pH 7.2 at 37 °C.
Physical form
Lyophilized powder containing 0.052% ammonium sulfate, 0.158% Tris−HCl, and 0.76% sodium chloride.
Storage Class
10 - Combustible liquids
wgk_germany
nwg
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Caspases: the executioners of apoptosis.
Cohen, G.M.
Biochemistry, 326 (part 1), 1-1 (1997)
Marcin Poreba et al.
Nature protocols, 12(10), 2189-2214 (2017-09-22)
Many biologically and chemically based approaches have been developed to design highly active and selective protease substrates and probes. It is, however, difficult to find substrate sequences that are truly selective for any given protease, as different proteases can demonstrate
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service