A8312
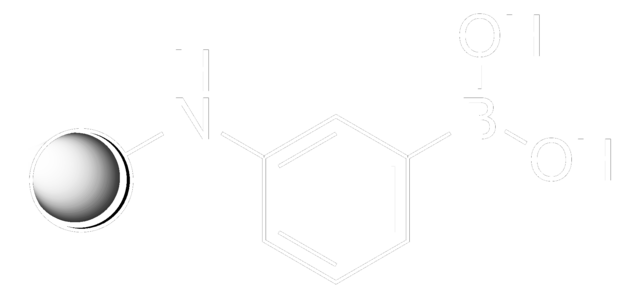
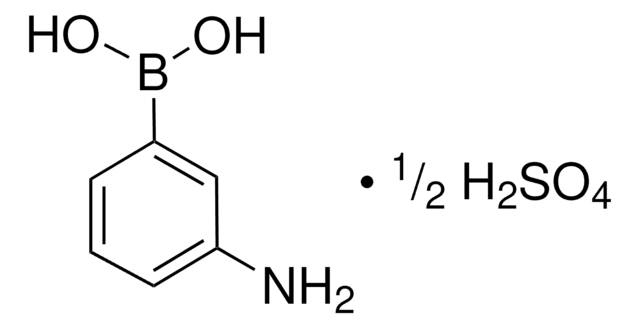
m-Aminophenylboronic acid–Agarose
aqueous suspension
Synonym(s):
(3-aminophenyl)boronic acid-Agarose, 3-Aminophenylboronic acid–Agarose, m-APBA-Agarose, m-Aminophenylboronic acid resin
About This Item
Recommended Products
form
aqueous suspension
Quality Level
extent of labeling
40-80 μmol per mL
matrix
6% beaded agarose
matrix activation
epichlorohydrin
matrix attachment
through amino to carboxyls of EDTA
matrix spacer
9 atoms
capacity
≥8 mg/mL binding capacity (peroxidase Type VI)
storage temp.
2-8°C
Looking for similar products? Visit Product Comparison Guide
General description
Application
- as a component of the column for the purification of Escherichia coli TOP10F cells
- with cell lysates of various cells for detection of PARylated (Poly(ADP-ribos)ylation) proteins.
- in the affinity purification of horseradish peroxidase (HRPO)
- in the glycated human serum albumin (gHSA) purification
Physical form
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service