A6936
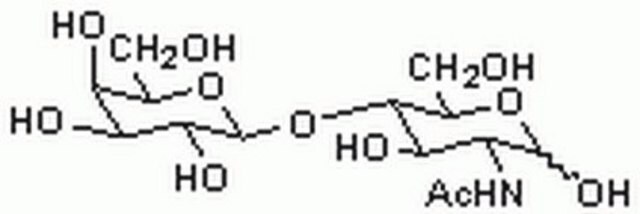
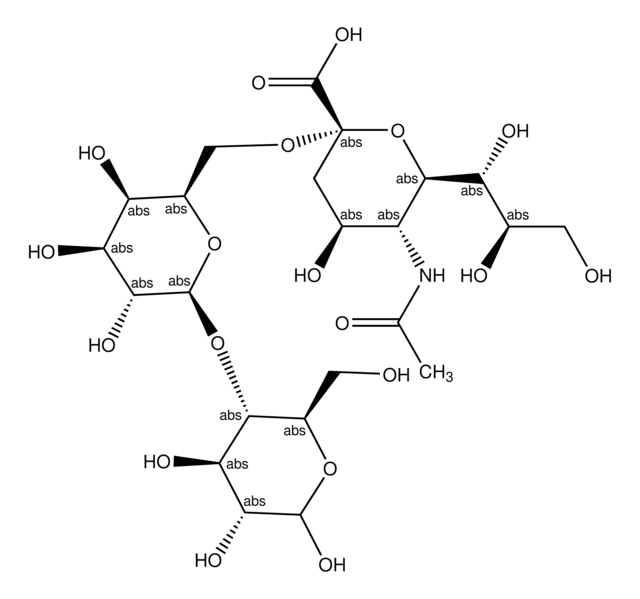
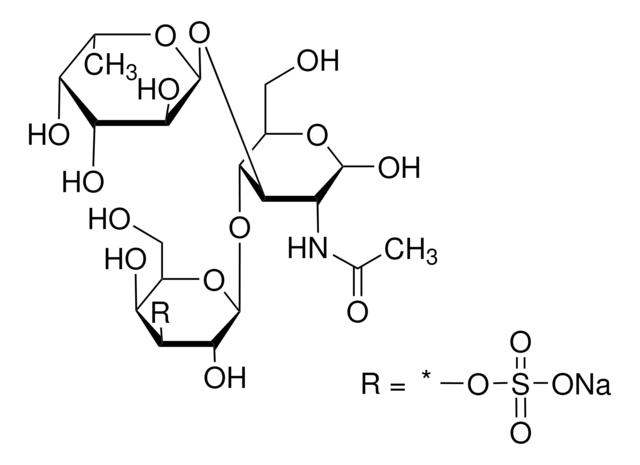
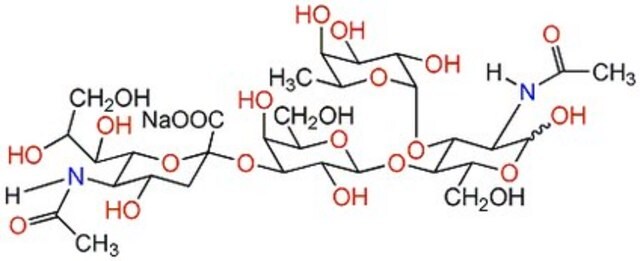
3′-N-Acetylneuraminyl-N-acetyllactosamine sodium salt
≥95%
Synonym(s):
α-NeuNAc-(2→3)-β-D-Gal-(1→4)-D-GlcNAc, 3′-SLN, 3′-Sialyl-N-acetyllactosamine
About This Item
Recommended Products
Quality Level
assay
≥95%
form
solid
storage temp.
−20°C
SMILES string
CC(=O)NC(C=O)C(O)C(OC1OC(CO)C(O)C(OC2(CC(O)C(NC(C)=O)C(O2)C(O)C(O)CO)C(O)=O)C1O)C(O)CO
InChI
1S/C25H42N2O19/c1-8(32)26-10(4-28)16(37)20(13(36)6-30)44-23-19(40)22(18(39)14(7-31)43-23)46-25(24(41)42)3-11(34)15(27-9(2)33)21(45-25)17(38)12(35)5-29/h4,10-23,29-31,34-40H,3,5-7H2,1-2H3,(H,26,32)(H,27,33)(H,41,42)
InChI key
RGZDLTASXRMKKF-UHFFFAOYSA-N
Application
Other Notes
signalword
Danger
hcodes
pcodes
Hazard Classifications
Acute Tox. 1 Oral
Storage Class
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
O-Glycans
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service