All Photos(3)
About This Item
Empirical Formula (Hill Notation):
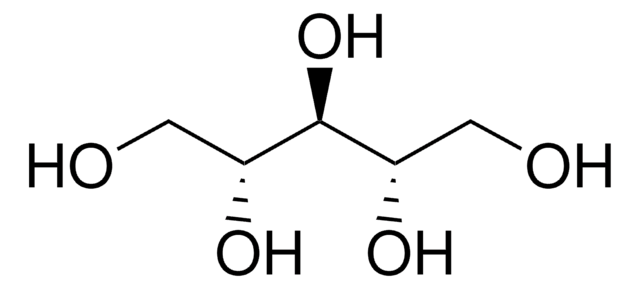
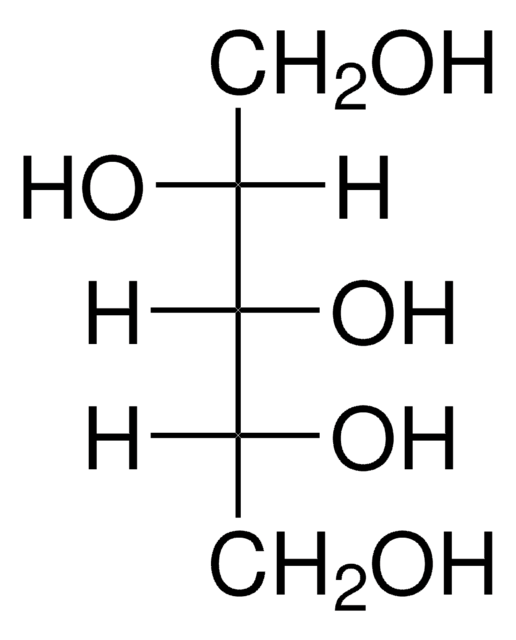
C5H12O5
CAS Number:
Molecular Weight:
152.15
Beilstein/REAXYS Number:
1720524
EC Number:
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
Quality Level
assay
≥99%
form
powder
color
white
mp
104 °C ((219 °F ))
solubility
water: 50 mg/mL, clear to slightly hazy, colorless to faintly yellow
SMILES string
OC[C@H](O)[C@H](O)[C@H](O)CO
InChI
1S/C5H12O5/c6-1-3(8)5(10)4(9)2-7/h3-10H,1-2H2/t3-,4+,5-
InChI key
HEBKCHPVOIAQTA-ZXFHETKHSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Adonitol (Ribitol), a pentose alcohol, is metabolized to teicholic acids used in the cell walls of gram positive bacteria. Adonitol is often compared to other cell permeating molecules such as formamide, propanediol, and DMSO as a cryopreservation agent.
Other Notes
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Anders Østergaard Madsen et al.
The journal of physical chemistry. A, 115(26), 7794-7804 (2011-06-15)
X-ray diffraction data of high quality measured to high resolution on crystals of the two pentitol epimers ribitol (centric) and xylitol (acentric) at 101, 141, and 181 K and data on the two compounds previously recorded at 122 K have
G Funke et al.
Journal of clinical microbiology, 31(11), 2907-2912 (1993-11-01)
Fifteen strains of CDC group 1 coryneform and biochemically similar bacteria were isolated from clinical specimens. Of the 15 strains isolated, 11 were derived from abscesses and purulent lesions, mostly from the upper part of the body, and 3 were
S Brisse et al.
Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases, 10(10), 942-945 (2004-09-18)
A rapid method combining gyrA PCR-restriction fragment length polymorphism analysis, parC PCR and adonitol fermentation was developed to identify Klebsiella pneumoniae phylogenetic groups KpI, KpII and KpIII. Analysis of 420 clinical isolates from 26 hospitals showed that the three groups
D J Brenner et al.
Journal of clinical microbiology, 15(4), 703-713 (1982-04-01)
DNA relatedness was used to define the biochemical boundaries of Escherichia coli. A large number of biochemically atypical strains were shown to belong to biogroups of E. coli. These included strains negative in reactions for indole, all three decarboxylases, D-mannitol
John Quiroga et al.
Frontiers in veterinary science, 8, 625347-625347 (2021-04-03)
Acute ruminal acidosis (ARA) occurs after an excessive intake of rapidly fermentable carbohydrates and is characterized by the overproduction of D-lactate in the rumen that reaches the bloodstream. Lameness presentation, one of the primary consequences of ARA in cattle, is
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service