85707
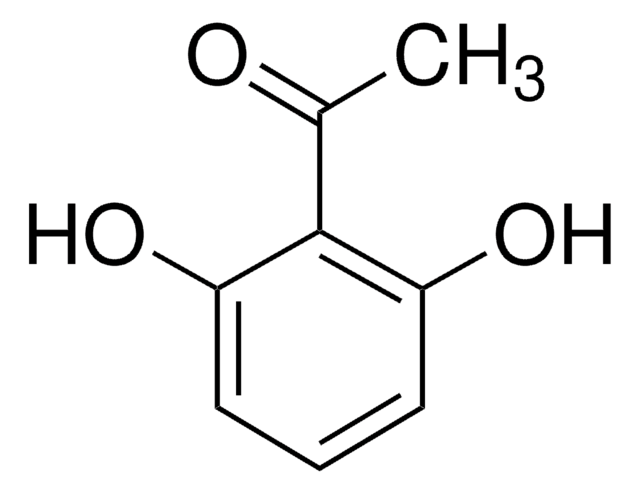
2,5-Dihydroxybenzoic acid
matrix substance for MALDI-MS, >99.0% (HPLC)
Synonym(s):
2,5-DHBA, DHB, Gentisic acid, Hydroquinonecarboxylic acid
About This Item
Recommended Products
grade
matrix substance for MALDI-MS
Quality Level
assay
>99.0% (HPLC)
form
powder
analyte functional class(es)
polymers
analyte chemical class(es)
glycans, lipids, organic molecules, peptides, proteins
technique(s)
MALDI-MS: suitable
mp
200-207 °C
204-208 °C (lit.)
cation traces
Ba: ≤5 mg/kg
Ca: ≤5 mg/kg
Cd: ≤5 mg/kg
Co: ≤5 mg/kg
Cr: ≤5 mg/kg
Cu: ≤5 mg/kg
K: ≤5 mg/kg
Mg: ≤5 mg/kg
Mn: ≤5 mg/kg
Na: ≤5 mg/kg
Ni: ≤5 mg/kg
Pb: ≤5 mg/kg
Zn: ≤5 mg/kg
ε (extinction coefficient)
>2000 at 337 nm
SMILES string
OC(=O)c1cc(O)ccc1O
InChI
1S/C7H6O4/c8-4-1-2-6(9)5(3-4)7(10)11/h1-3,8-9H,(H,10,11)
InChI key
WXTMDXOMEHJXQO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Related product
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Glycosylation is known to have profound influence on various physiochemical, cellular and biological functions of proteins. Alterations in this modification are known to affect the immune system and have been associated with various pathological states such as cancer, rheumatoid arthritis, and inflammatory diseases.
Glycosylation is known to have profound influence on various physiochemical, cellular and biological functions of proteins. Alterations in this modification are known to affect the immune system and have been associated with various pathological states such as cancer, rheumatoid arthritis, and inflammatory diseases.
Mass Spectrometry of Glycans, method comparison and products
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![trans-2-[3-(4-tert-Butylphenyl)-2-methyl-2-propenylidene]malononitrile matrix substance for MALDI-MS, ≥99.0% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/249/587/f8021369-f65a-413d-887d-3c8a4d2a248f/640/f8021369-f65a-413d-887d-3c8a4d2a248f.png)