199443
Aluminum oxide
standard grade, Brockmann I, activated, basic
Synonym(s):
Alumina
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
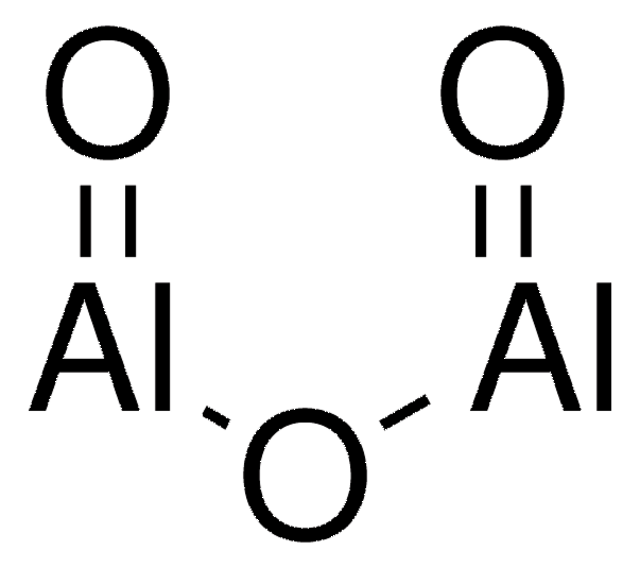
Al2O3
CAS Number:
Molecular Weight:
101.96
EC Number:
MDL number:
UNSPSC Code:
12352311
eCl@ss:
38120402
PubChem Substance ID:
NACRES:
SB.52
Recommended Products
agency
suitable for EPA 1613
Quality Level
form
powder
manufacturer/tradename
CAMAG 5016-A-I
technique(s)
solid phase extraction (SPE): suitable
impurities
<0.03% Fe2O3
<0.4% Na2O
particle size
~150 mesh
pore size
58 Å pore size
pH
9.5±0.5 ( in H2O)
mp
2040 °C (lit.)
anion traces
chloride (Cl-): none detected
silicate (as SiO2): <0.03%
SMILES string
O=[Al]O[Al]=O
InChI
1S/2Al.3O
InChI key
TWNQGVIAIRXVLR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Brockmann aluminum oxides are suitable for column chromatography and adsorptive filtration.
The product is basic activated aluminum oxide (Alumina, Al2O3) having an activity grade Brockman I. The mechanism of formation of protective scales of α-Al2O3 on surfaces of binary M-Al (M = Fe, Ni or Co) alloys at high temperatures has been studied. Activated aluminum oxides having different acidities and water concentrations (having Brockmann activity I-V) can easily detect various types of water (namely free water, pore water, and bound water) on the aluminum oxide present in soils.
Application
Aluminum oxide (Alumina) has been used for the removal of peroxides from the dehydration solvent (tetrahydrofuran, THF) in a protocol. Protocol involved the high resolution study of fine brain anatomy of fluorescently labeled mouse brains.
It may be used in the fabrication of polycrystalline lithium iodide-aluminum oxide solid electrolytes. The incorporation of aluminum oxide remarkably increased the conductivity of lithium iodide.
It may be used in the fabrication of polycrystalline lithium iodide-aluminum oxide solid electrolytes. The incorporation of aluminum oxide remarkably increased the conductivity of lithium iodide.
Storage Class
11 - Combustible Solids
wgk_germany
nwg
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Micron-scale resolution optical tomography of entire mouse brains with confocal light sheet microscopy.
Silvestri L, et al.
Journal of Visualized Experiments, 80, e50696-e50696 (2013)
Conduction Characteristics of the Lithium Iodide-Aluminum Oxide Solid Electrolytes.
Liang CC.
Journal of the Electrochemical Society, 120(10), 1289-1292 (1973)
Thermoanalytical studies of water on activated alumina, Brockmann I-V,(acid, neutral, basic) from- 60? to+ 700? C.
Hampson JW and Bleam WF.
Thermochimica Acta, 288(1), 179-189 (1996)
Prescott R and Graham MJ.
Oxidation of Metals, 38(3-4), 233-254 (1992)
Chien-Chih Lin et al.
Nanoscale, 5(17), 8090-8097 (2013-07-25)
We demonstrated a promising route for enhancing temperature sensitivity, improving saturation voltage, and reducing power consumption of the MOS(p) tunneling temperature sensors by introducing ultrathin Al2O3 into the dielectric stacks. Detailed illustrations of the working mechanism and device concept are
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 199443-100G | 4061835561612 |
| 199443-1KG | 4061838763204 |
| 199443-20KG | 4061838763211 |
| 199443-5G | 4061832852966 |
| 199443-5KG | 4061838763228 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service