PHR1784
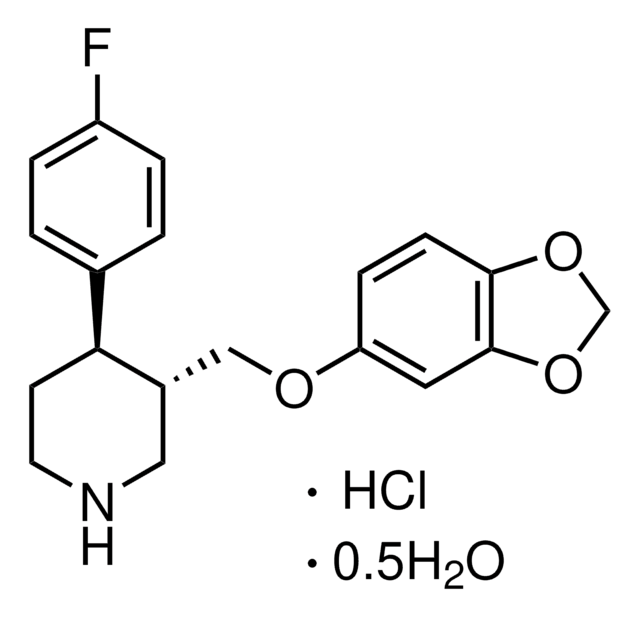
Aripiprazole
Pharmaceutical Secondary Standard; Certified Reference Material
Synonym(s):
Aripiprazole, 7-{4-[4-(2,3-Dichlorophenyl)-1-piperazinyl]butoxy}-3,4-dihydro-2(1H)-quinolinone
About This Item
Recommended Products
grade
certified reference material
pharmaceutical secondary standard
Quality Level
agency
traceable to Ph. Eur. Y0001649
traceable to USP 1042634
API family
aripiprazole
CofA
current certificate can be downloaded
packaging
pkg of 500 mg
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-30°C
InChI
1S/C23H27Cl2N3O2/c24-19-4-3-5-21(23(19)25)28-13-11-27(12-14-28)10-1-2-15-30-18-8-6-17-7-9-22(29)26-20(17)16-18/h3-6,8,16H,1-2,7,9-15H2,(H,26,29)
InChI key
CEUORZQYGODEFX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Aripiprazole is a benzisoxazole derivative and second-generation antipsychotic and anti-depressant drug. It is used in the management of schizophrenia and bipolar I disorder. It shows partial agonistic activity towards dopamine D2 and serotonin 5-HT1A receptors and antagonistic activity at 5-HT2A receptors.
Application
- Development of a reversed phase-high-performance liquid chromatography (RP-HPLC) method for the determination of aripiprazole in the presence of nine of its impurities in both bulk and its pharmaceutical formulations
- Direct voltammetric determination of aripiprazole in pharmaceutical formulations, human serum, and urine samples
- Simultaneous estimation of aripiprazole and octoclothepin in pharmaceutical formulations and human urine samples by two different voltammetric methods using glassy carbon electrodes
- UV-spectroscopic determination of aripiprazole in pure and tablet formulations following ICH guidelines
- Multi-analysis of aripiprazole, clozapine, and sulpiride in pharmaceutical formulations and biological fluids by extraction-free UV-Visible spectrophotometric methods based on the ability of the three antipsychotic drugs to form stable ion-pair complexes with bromophenol blue (BPB) and bromothymol blue (BTB)
Analysis Note
Footnote
Recommended products
Related product
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service