P2920000
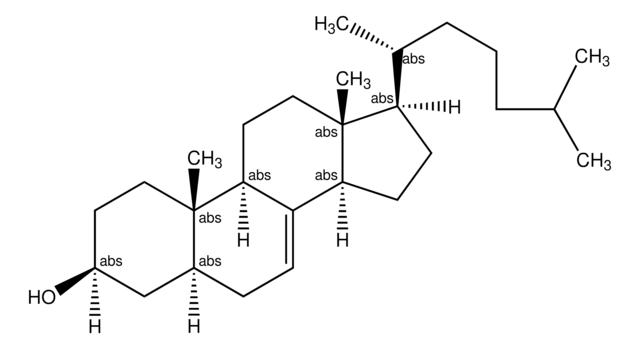
Pregnenolone isobutyrate
European Pharmacopoeia (EP) Reference Standard
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C25H38O3
Molecular Weight:
386.57
UNSPSC Code:
41116107
NACRES:
NA.24
Recommended Products
grade
pharmaceutical primary standard
API family
pregnenolone
manufacturer/tradename
EDQM
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-8°C
General description
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support ofthis product, including SDS and any product information leaflets, has been developed and issued under the Authority of the issuing Pharmacopoeia.
Application
This European Pharmacopoeia reference standard is intended for use only as specifically prescribed in the European Pharmacopoeia. Their suitability for any other use is not guaranteed and is the sole responsibility of the user. This standard is not intended for human or animal use.Established for the preparation of internal standard solution in the assay of:
- Cholesterol
- Cholesterol for parenteral use
- Wool alcohols
Packaging
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
Other Notes
Sales restrictions may apply.
Related product
Product No.
Description
Pricing
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Cholesterol
European Pharmacopoeia Commission and European Directorate for the Quality of Medicines & Healthcare
European pharmacopoeia, 2201-2202 (2020)
Cholesterol for parenteral use
European Pharmacopoeia Commission and European Directorate for the Quality of Medicines & Healthcare
European pharmacopoeia, 6111-6112 (2022)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service