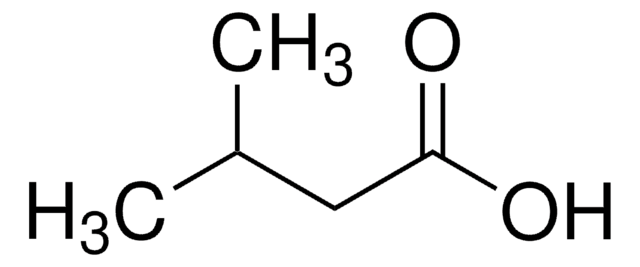
75054
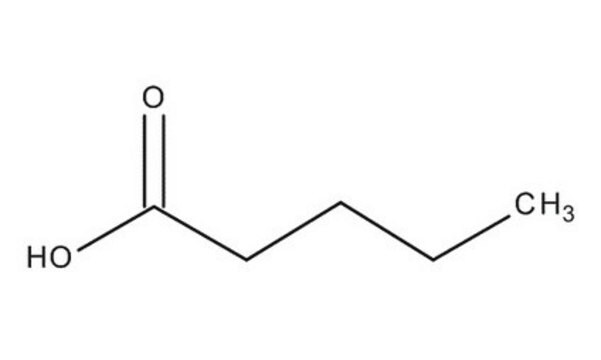
Valeric acid
analytical standard
Synonym(s):
n-Valeric acid, Pentanoic acid
About This Item
Recommended Products
grade
analytical standard
Quality Level
vapor density
3.5 (vs air)
vapor pressure
0.15 mmHg ( 20 °C)
assay
≥99.8% (GC)
autoignition temp.
707 °F
shelf life
limited shelf life, expiry date on the label
expl. lim.
7.6 %
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
refractive index
n20/D 1.408 (lit.)
n20/D 1.408
bp
110-111 °C/10 mmHg (lit.)
185 °C (lit.)
mp
−20-−18 °C (lit.)
density
0.939 g/mL at 25 °C (lit.)
application(s)
cleaning products
cosmetics
flavors and fragrances
food and beverages
personal care
format
neat
SMILES string
CCCCC(O)=O
InChI
1S/C5H10O2/c1-2-3-4-5(6)7/h2-4H2,1H3,(H,6,7)
InChI key
NQPDZGIKBAWPEJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
signalword
Danger
hcodes
Hazard Classifications
Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 1
flash_point_f
192.2 °F - closed cup
flash_point_c
89 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Separation of Propionic acid; Acetic acid; Heptanoic acid; Isobutyric acid; Valeric acid; Isocaproic acid; Butyric acid; Isovaleric acid
Separation of Methyl oleate; Caprylic acid; Heptanoic acid; Methyl decanoate; Methyl dodecanoate; Myristic acid; Methyl palmitate; Methyl palmitoleate; Methyl stearate; Methyl linoleate; Methyl linolenate; Acetic acid; Arachidic acid; Behenic acid; Propionic acid; Isobutyric acid; Valeric acid; Isovaleric acid; Isocaproic acid; Butyric acid
Protocols
In this study, SPME was used for the analysis of free fatty acids in Parmesan cheese using a 65 μm Carbowax/divinylbenzene (DVB) SPME fiber. Headspace extraction of the cheese sample was conducted at 65 °C for 15 minutes and analyzed by GC with FID detection. SPME is ideal for analyzing the volatiles associated with solid food samples. The phase chemistry of the Nukol GC column provides excellent peak shape of acidic compounds.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service