General description
Glutamate receptor 2 (UniProt P19491; also known as AMPA-selective glutamate receptor 2, GluA2, GluR-2, GluR-B, GluR-K2, GluR2, Glutamate receptor ionotropic, AMPA 2) is encoded by the Gria2 (also known as Glur2) gene (Gene ID 29627) in rat species. Glutamate receptors are synaptic receptors located primarily on the membranes of neuronal cells. These receptors play important roles in neural communication, memory formation, learning, and regulation by mediating glutamate-dependent postsynaptic excitation. Glutamate receptors are divided into two types, ionotropic glutamate receptors (iGluRs) and metabotropic glutamate receptors (mGluRs). iGluRs form ion channel pores upon glutamate binding, while mGluRs indirectly activate ion channels on the plasma membrane via G protein-coupled signaling cascade. Although all iGluRs and mGluRs bind glutamate, they do exhibit different binding activities toward chemical ligands, such as NMDA, kainate, AMPA, L-AP4, ACPD, L-QA, and such distinciton is used to divide these receptors further into subtypes. Glutamate receptor 2 is an AMPA type iGluR, GluR-1 is initially produced with an N-terminal signal peptide sequence (a.a. 1-24), the removal of which yields the mature receptor with 4 transmembrane helices (a.a. 544-564, 592-610, 617-637, 813-833), having a large N-terminal extracellular domain (a.a. 24-543), followed by two intracellular loops (a.a. 565-591, 611-616), one extracellular loop (a.a. 638-812) and a C-terminal cytoplasmic tail (a.a. 834-883).
Specificity
Clone 14C12.2 targets an epitope within the N-terminal extracellular domain present in all spliced isoforms of human, mouse, and rat GluR2 reported by UniProt (P42262, P23819, P19491).
Immunogen
GST-tagged recombinant rat GluR2 N-terminal extracellular domain fragment.
Application
Anti-GluR2, clone 14C12.2, Cat. No. MABN1189, is a highly specific mouse monoclonal antibody, that targets Glutamate Receptor 2 and has been tested in Western Blotting and Immunohistochemistry (Paraffin).
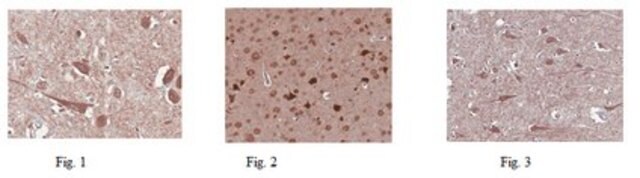
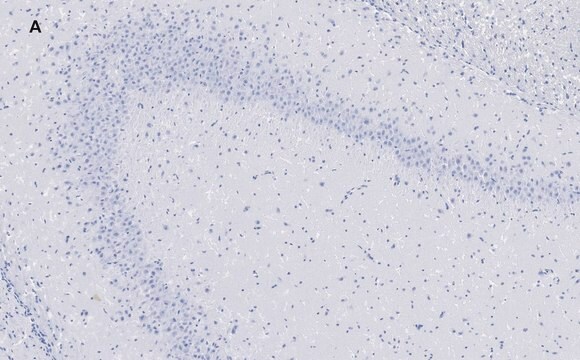
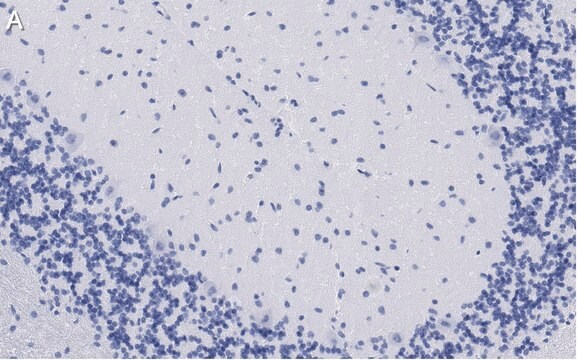
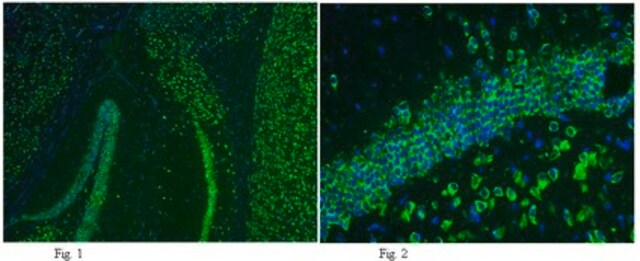
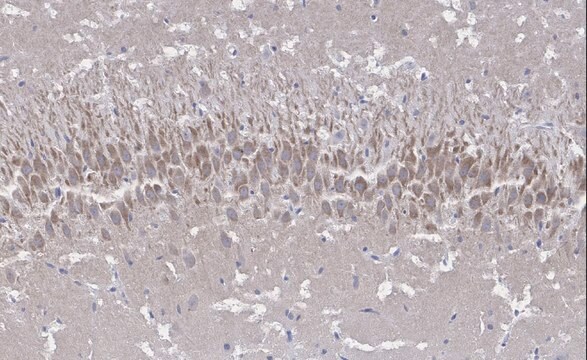
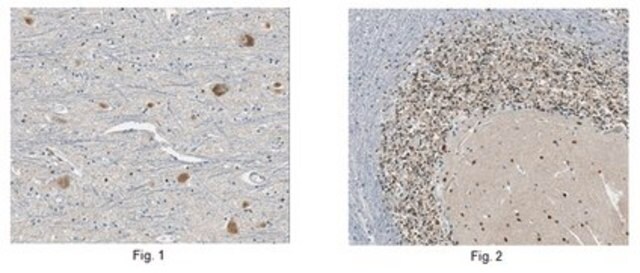
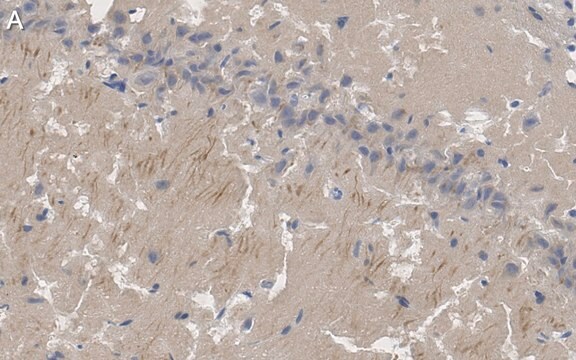
Immunohistochemistry Analysis: A 1:50-1,000 dilution from a representative lot detected GluR2 in mouse (hippocampus) and human (cerebellum and cerebral cortex) brain tissue sections.
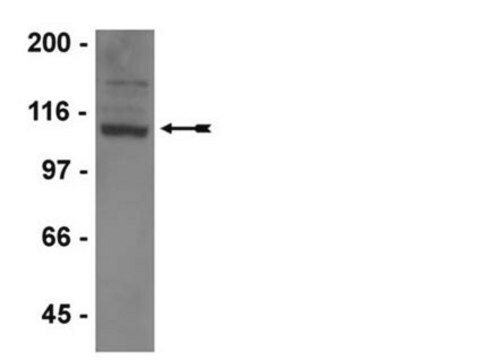
Western Blotting Analysis: 0.5 µg/mL of this antibody detected GluR2 in 10 µg of rat brain membrane extract.
Research Category
Neuroscience
Quality
Evaluated by Western Blotting in mouse brain membrane extract.
Western Blotting Analysis: 0.5 µg/mL of this antibody detected GluR2 in 10 µg of mouse brain membrane extract.
Target description
~110 kDa observed. Target band size appears larger than the caluclated molecular weights (signal peptide removed) of 96.18/96.24/97.96/93.78 kDa (human isoform Flop/Flip/3/4), 96.02/83.54/101.9/100.2 kDa (mouse isoform 1/2/3/4), 90.05/96.11/97.78 kDa (rat isoform Flop/Flip/3) due to glycosylation. Uncharacterized bands may be observed in some lysate(s).
Physical form
Format: Purified
Protein G purified.
Purified mouse IgG2a in buffer containing 0.1 M Tris-Glycine (pH 7.4), 150 mM NaCl with 0.05% sodium azide.
Storage and Stability
Stable for 1 year at 2-8°C from date of receipt.
Other Notes
Concentration: Please refer to lot specific datasheet.
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.